Question
Question: Benzene can be obtained by heating either benzoic acid with X or phenol with Y. X and Y are ________...
Benzene can be obtained by heating either benzoic acid with X or phenol with Y. X and Y are ________ respectively.
A. zinc dust and soda lime
B. soda lime and zinc dust
C. zinc dust and sodium hydroxide
D. soda lime and copper
Solution
Phenol to benzene is a radical reaction. While benzoic acid to benzene is a decarboxylation reaction. This occurs at low temperature and high temperature.
Complete step by step answer:
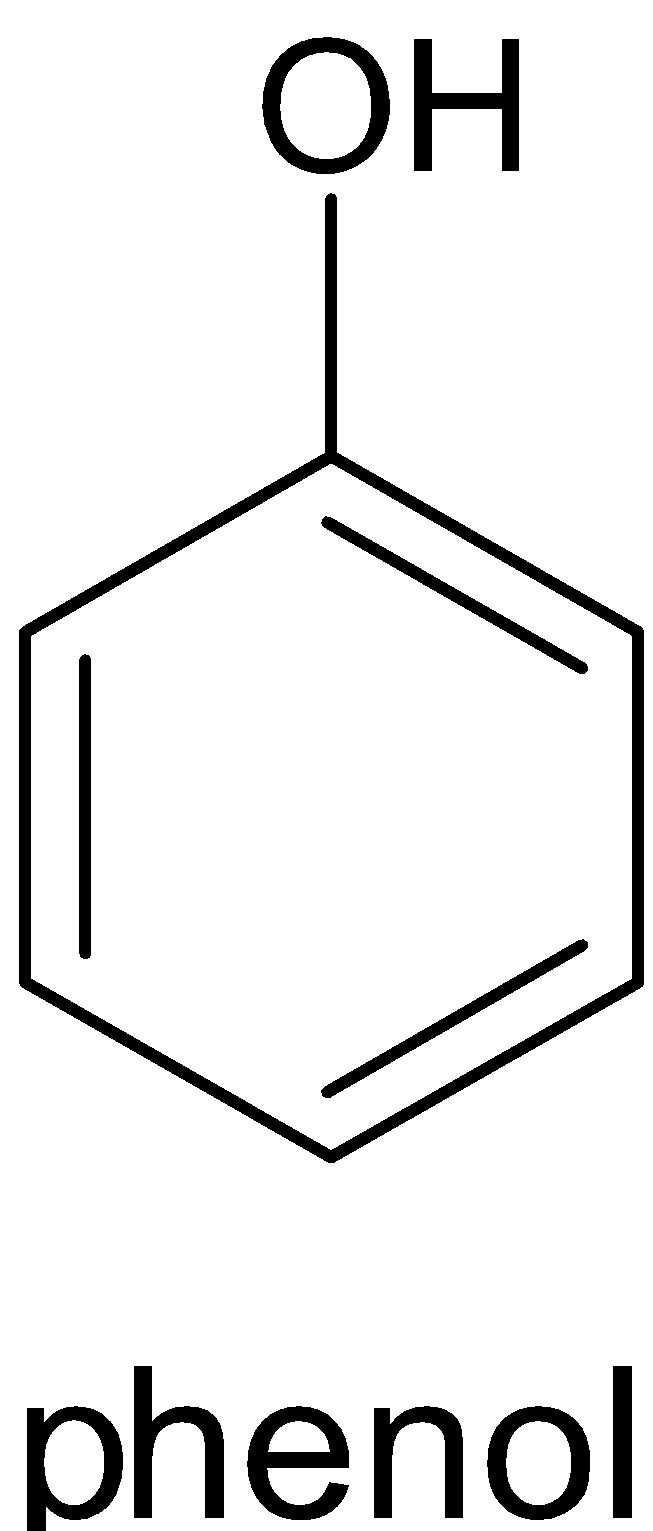 Y
Y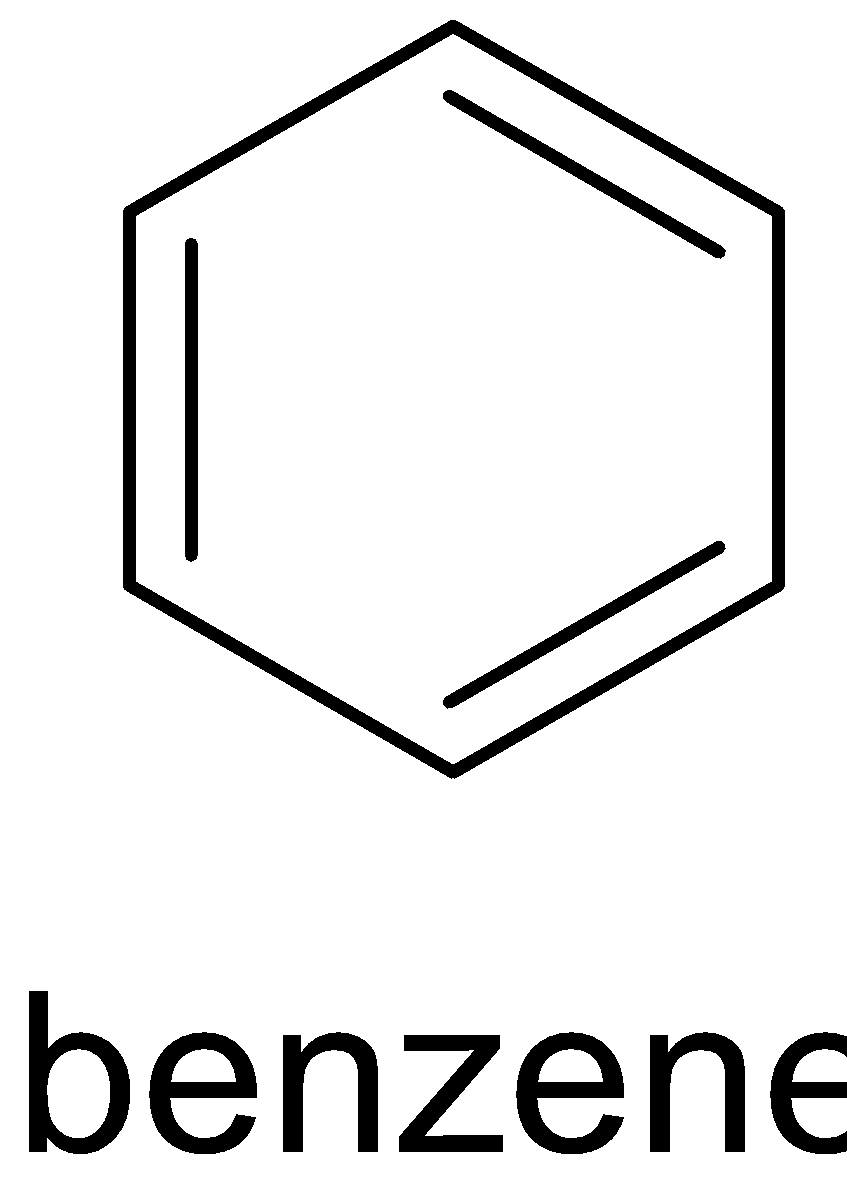
This reaction can only be done by heating phenol with zinc dust. The product formed will be benzene and zinc oxide. When phenol reacts with zinc dust, it releases proton which forms phenoxide ion. The proton then accepts an electron from zinc and produces a hydrogen radical and zinc cation. When phenoxide ion is heated, it forms phenyl radical. This phenyl radical reacts with the hydrogen radical and forms benzene. The complete reaction is given below:
C6H5OH→C6H5O−+H+
H++Zn→H∙+Zn+
C6H5O−ΔC6H5∙+O∙−
C6H5∙+H∙→C6H6
Zn++O−→Zn2++O2−→ZnO
Zinc dust oxidizes itself to ZnO and reduces phenol to benzene.
Therefore Y is zinc dust.
Soda lime is the mixture of NaOH and CaO. Reaction of benzoic acid with soda lime is called Oakwood reaction. Sodium hydroxide removes CO2 in benzoic acid molecules which produce alkane, i.e benzene. And the CO2 reacts with sodium hydroxide to give sodium carbonate. The function of CaO is that it makes NaOH less reactive. The reaction is given below:
$$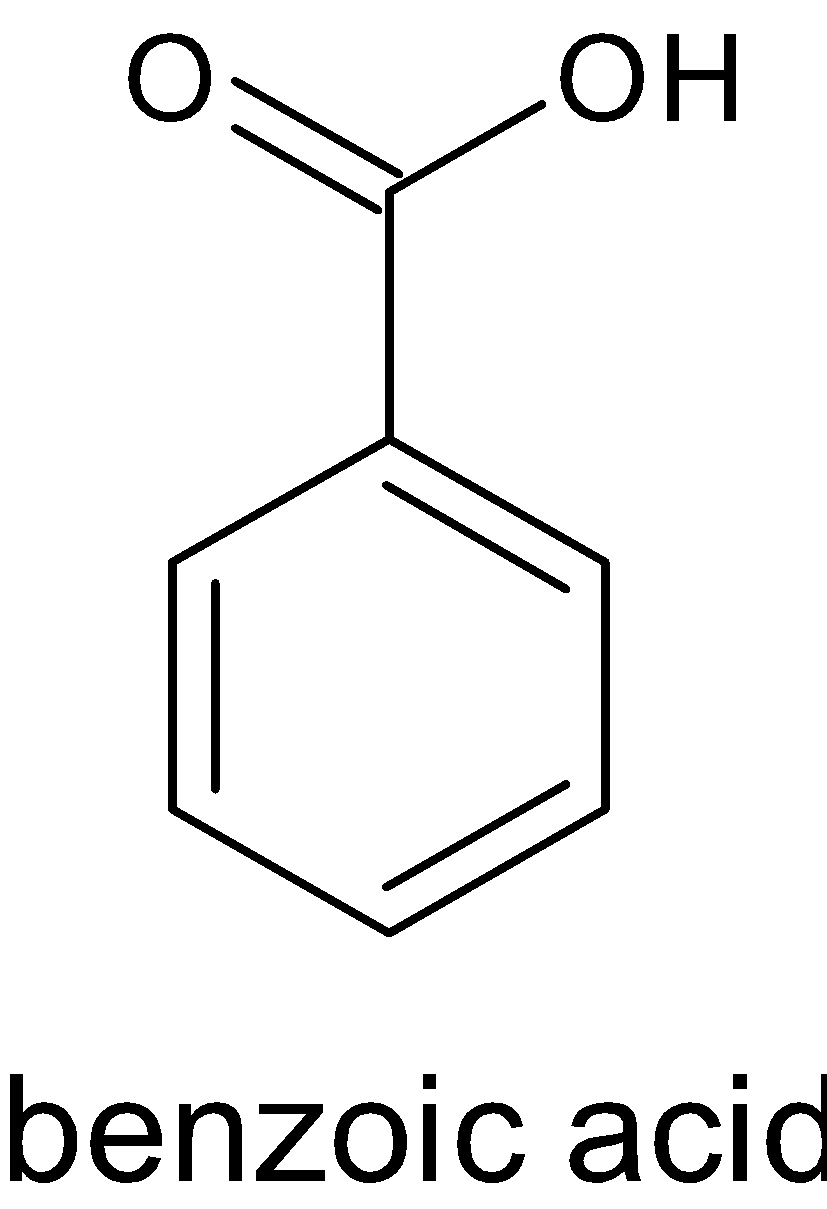 +NaOH+CaO→
+NaOH+CaO→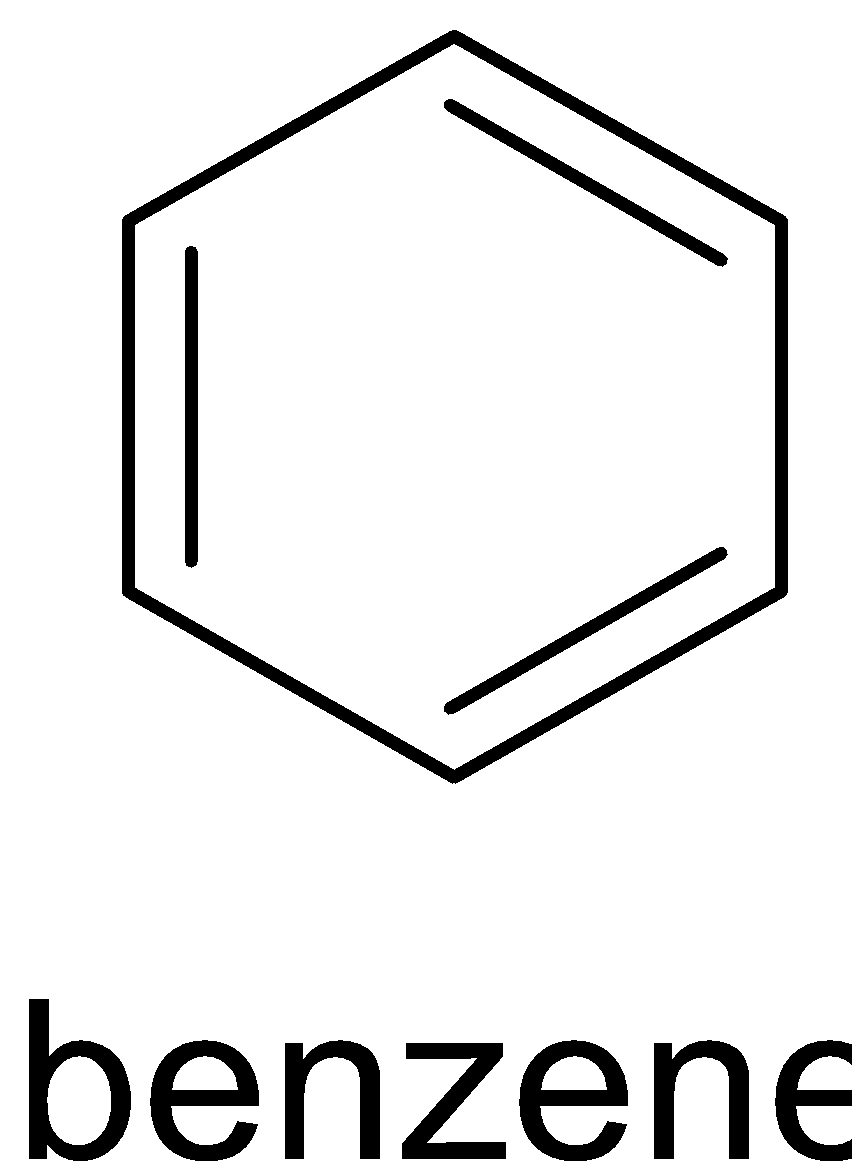 +Na2CO3
+Na2CO3
Hence, X is soda lime and Y is zinc dust.
So, the correct answer is option B.
Additional information Since CO2 is removed, it is called a decarboxylation reaction. Soda lime is also used for decarboxylation of sodium salts of aromatic compounds. This reaction is called the Duma reaction.
Note:
At lower temperatures, when soda lime is used, benzoic acid forms sodium benzoate and then forms benzene and sodium carbonate. At higher temperatures, it directly forms benzene. This reaction is similar to pyrolysis.
