Question
Question: Benzene can be conveniently converted into n-propyl benzene by: A.Friedel-craft alkylation with n-...
Benzene can be conveniently converted into n-propyl benzene by:
A.Friedel-craft alkylation with n-propyl chloride.
B.Friedel-Craft acylation with propionyl chloride followed by Wolff-Kishner reduction.
C.Friedel-Craft acylation with propionyl chloride followed by catalytic hydrogenation.
D.Friedel-Craft acylation with propionyl chloride followed by reduction with LiAlH4
Solution
Preparation of n-propylbenzene from Benzene is a two-step process. We cannot prepare n-propylbenzene from benzene in a single step. In the first step benzene reacts with propionyl chloride and then undergoes reduction with hydrazine.
Complete answer:
The preparation of n-propyl benzene from benzene is as follows.
Step-1: Friedel-craft acylation:
Reaction of Benzene with propionyl chloride is called Friedel-craft acylation reaction. Because the acyl group is going to be added to benzene.
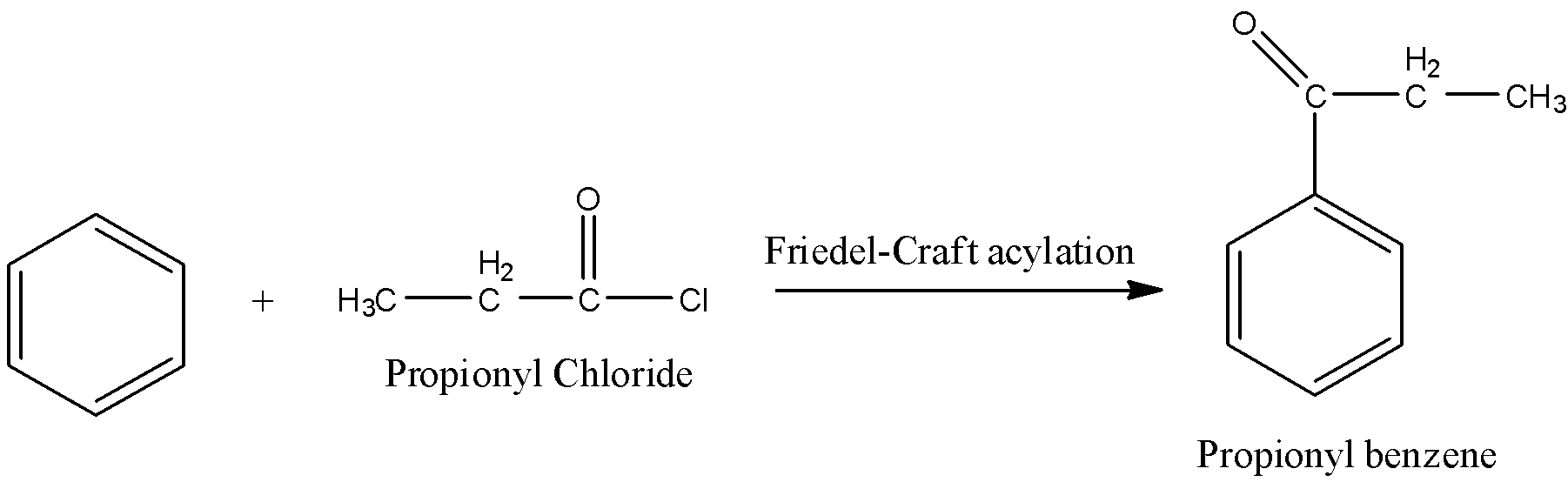
Step-2: Wolff-Kishner reduction:
In this reaction propionyl benzene reacts with hydrazine and forms a hydrazine derivative. This reaction is called Wolff-Kishner reduction.
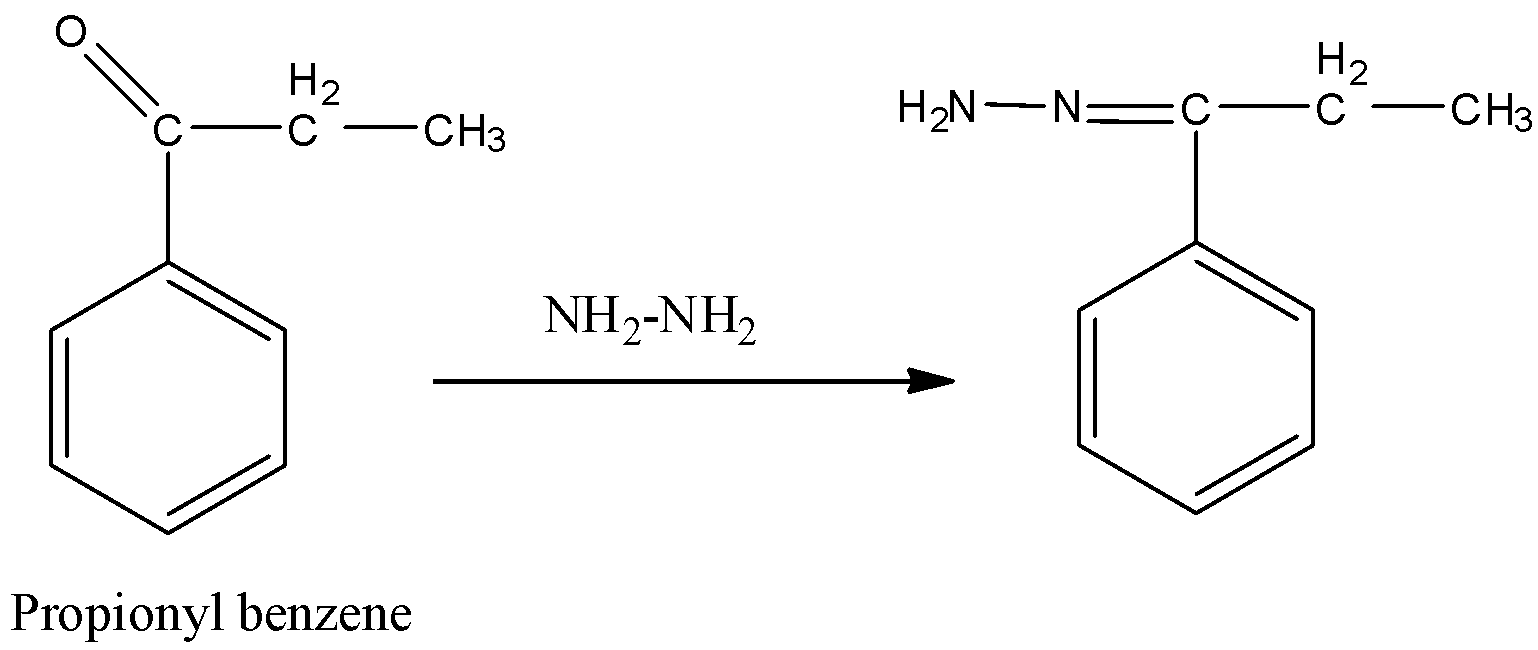
Followed by Wolff-Kishner reduction, reaction with KOH, ethylene glycol forms n-propyl benzene.
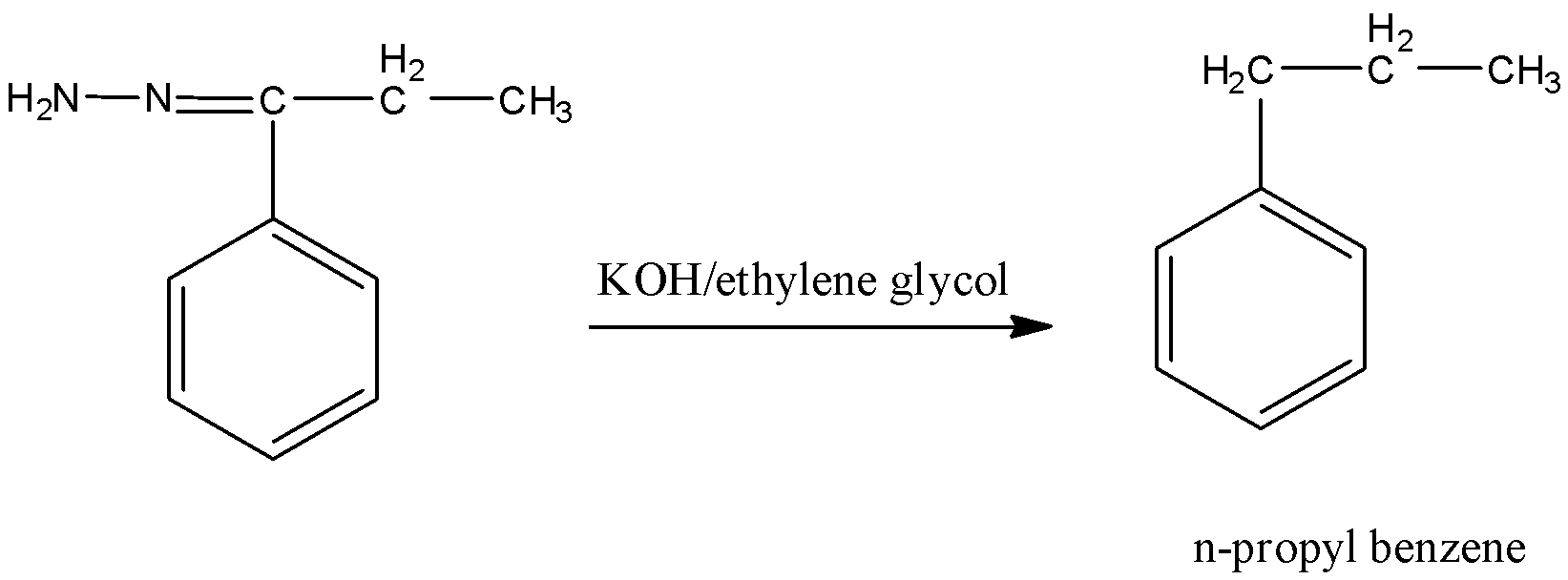
Therefore, the preparation of n-propyl benzene from benzene involves Friedel-Craft acylation with propionyl chloride followed by Wolff-Kishner reduction.
So, the correct option is B.
Note:
We are not supposed to use catalytic hydrogenation or reduction with lithium aluminum hydride (LiAlH4) in step-2. Because catalytic hydrogen or reduction with aluminum hydride (LiAlH4) may cause reduction on the benzene ring. So, we are not supposed to use these reducing agents in the step-2. Wolff-Kishner reduction is the best way to reduce carbonyl carbon without disturbing the structure of the benzene.
