Question
Question: Benzamide on treatment with \({\text{POC}}{{\text{l}}_{\text{3}}}\)gives: A. Aniline B. Benzonit...
Benzamide on treatment with POCl3gives:
A. Aniline
B. Benzonitrile
C. Chlorobenzene
D. Benzyl amine
Solution
In the above question, it is asked what happened when benzamide is treated with POCl3. Since, POCl3 is a dehydrating agent, it dehydrates benzamide to form a cyanide.
Complete step-by-step answer: Phosphoryl chloride is a colourless liquid with the formula POCl3. It hydrolyses in moist air and releases phosphoric acid and fumes of hydrogen chloride. POCl3 is a commonly used dehydrating agent in chemical laboratories. It is used as a dehydrating agent for the preparation of nitriles from primary amides.
Benzamide is a white solid with chemical formula C6H5CONH2. It is the simplest amide derived from benzoic acid. It is soluble in organic solvents and water. These are organic compounds which contain a carboxamido substituent which is attached to a benzene ring. Benzamide is an extremely weak basic compound.
When benzamide reacts with POCl3, dehydration reaction take place, which means H2O is lost from the compound which results in the formation of benzonitrile. Benzonitrile is used as an intermediate for rubber chemicals and as a solvent for nitrile rubber.
The reaction of conversion of benzamide on treatment with POCl3 to give benzonitrile is given below:
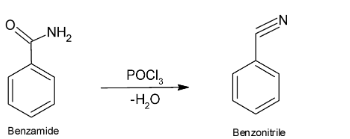
So, we can say that Benzonitrile is formed when benzamide reacts with POCl3.
Hence, the correct option is option B.
Note: Benzo nitriles react with amine to form N-substituted benzamide after hydrolysis.
Benzo nitriles ligands are readily displaced by stronger ligands making benzo nitriles complexes useful synthetic intermediates.
Nitrile-hydrolysing enzymes were generally unstable for commercial use in the isolated or purified state.
