Question
Question: Benzaldoxime can exist in two geometrical isomeric forms known as: A. cis and trans B. dextro an...
Benzaldoxime can exist in two geometrical isomeric forms known as:
A. cis and trans
B. dextro and leavo
C. syn and anti
D. E and Z
Solution
If an organic compound or chemical contains a double in it then the organic compound tries to exhibit either cis and trans form otherwise there is a chances of showing syn and anti or E and Z configuration.
Complete answer:
- In the question it is asked which geometrical isomerism is going to be exhibited by the benzaldoxime chemical.
- First we should know the structure of the given chemical to know which geometrical isomerism is going to be exhibited by the benzaldoxime.
- In the chemical name it is clearly mentioned that there is a presence of oxime functional group (=N-OH)
- The structure of the benzaldoxime is as follows.
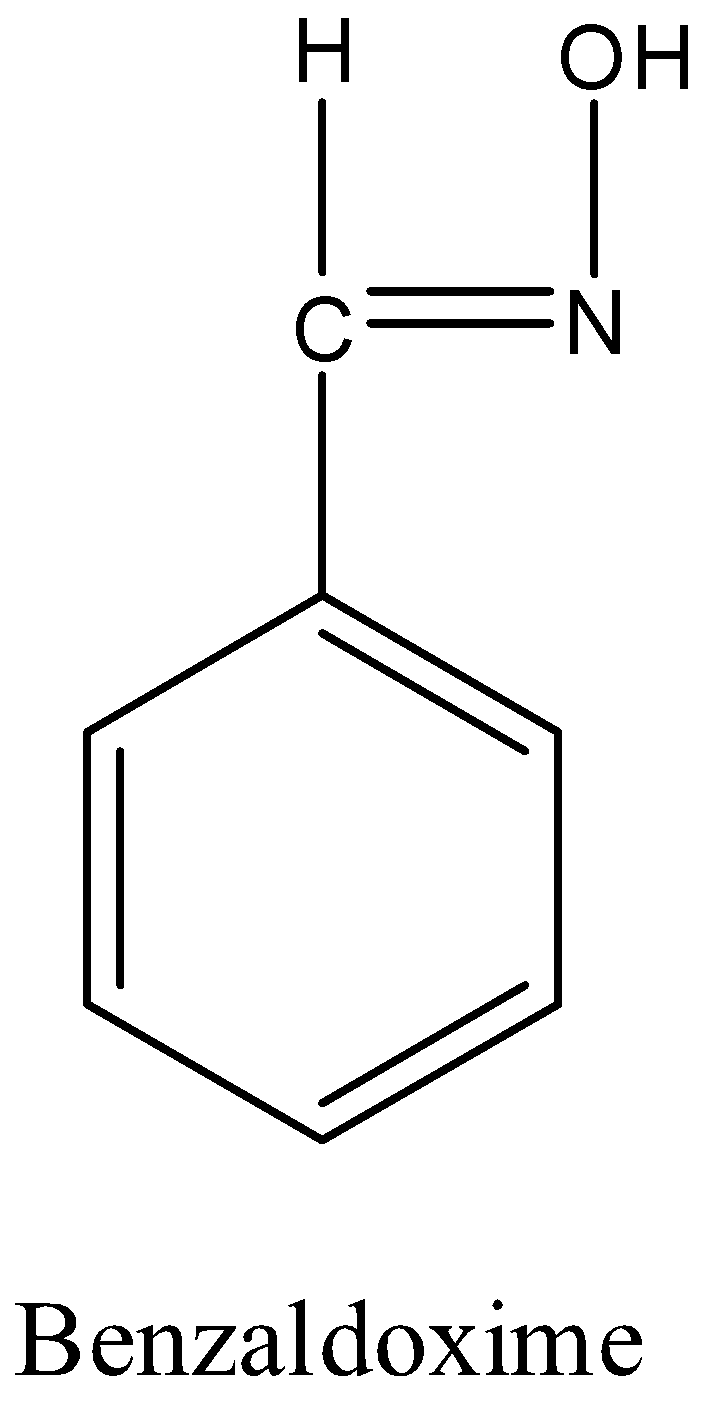
- We can see the functional group oxime in the above chemical structure.
- The chemical benzaldoxime is going to exist in the following form.
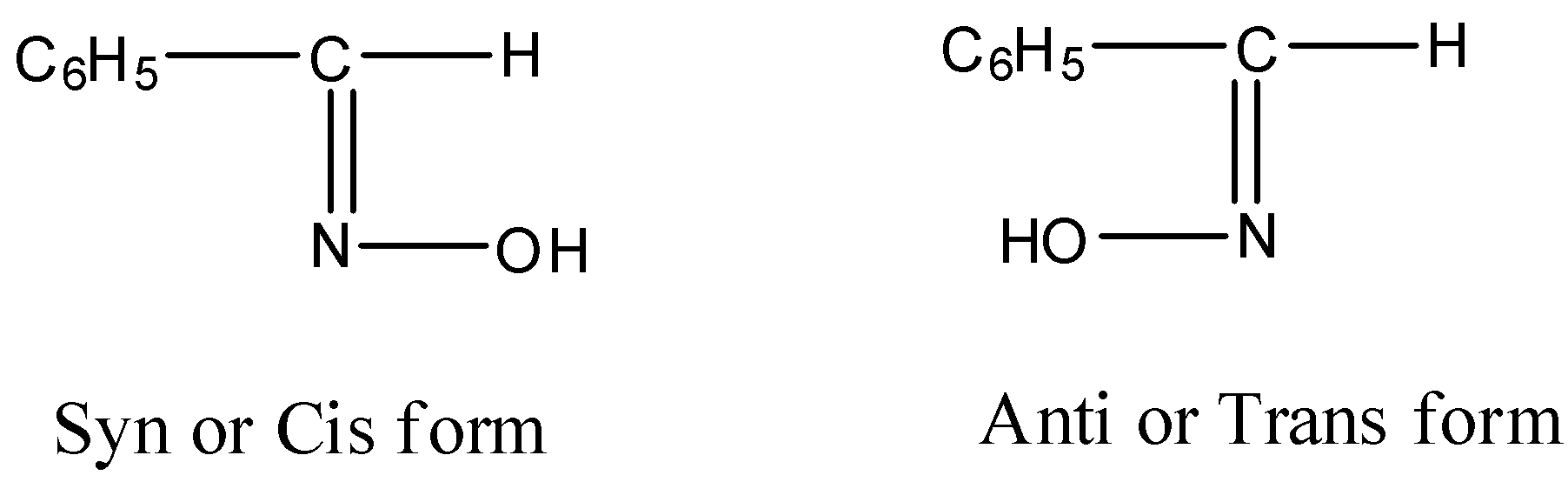
- By observing the above structures we can say that the benzaldoxime is going to exhibit two types of geometrical isomerism called Cis and Trans and syn and anti.
Therefore the options A and B are suitable for the structure of the benzaldoxime.
Note:
Generally oxime functional groups are going to exhibit geometrical isomerism in the form of syn and anti-forms due to the presence of the hydroxyl group on the nitrogen. Because of the presence of the hydroxyl group two types of geometrical isomerism called Cis and Trans and syn and anti are going to exist.
