Question
Question: Benzaldehyde to \[\alpha \]-hydroxyphenylacetic acid....
Benzaldehyde to α-hydroxyphenylacetic acid.
Solution
The structures of Benzaldehyde and α-hydroxyphenylacetic acid are as follows. So as we can see that one carbon has increased in the chain and that carbon is actually a carboxylic acid. We can use nucleophilic addition reactions to add a carbon atom in the aldehyde.
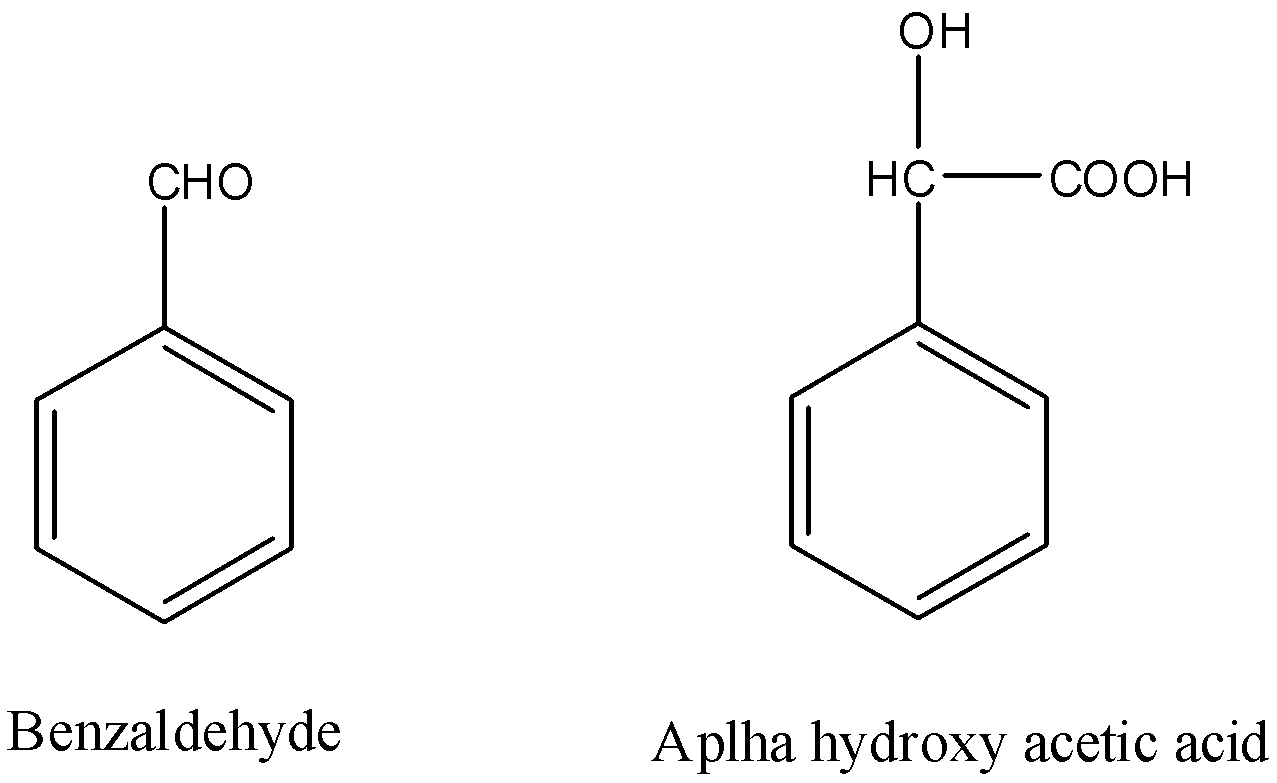
Complete step by step solution:
-The preparation of α-hydroxyphenylacetic acid from benzaldehyde contains two steps.
Step-1:
-Benzaldehyde undergoes nucleophilic addition reaction with sodium cyanide (NaCN) in the presence of hydrochloric acid and gives benzaldehyde cyanohydrin as the product at pH 9-10. Conversion of benzaldehyde to benzaldehyde cyanohydrin is as follows.
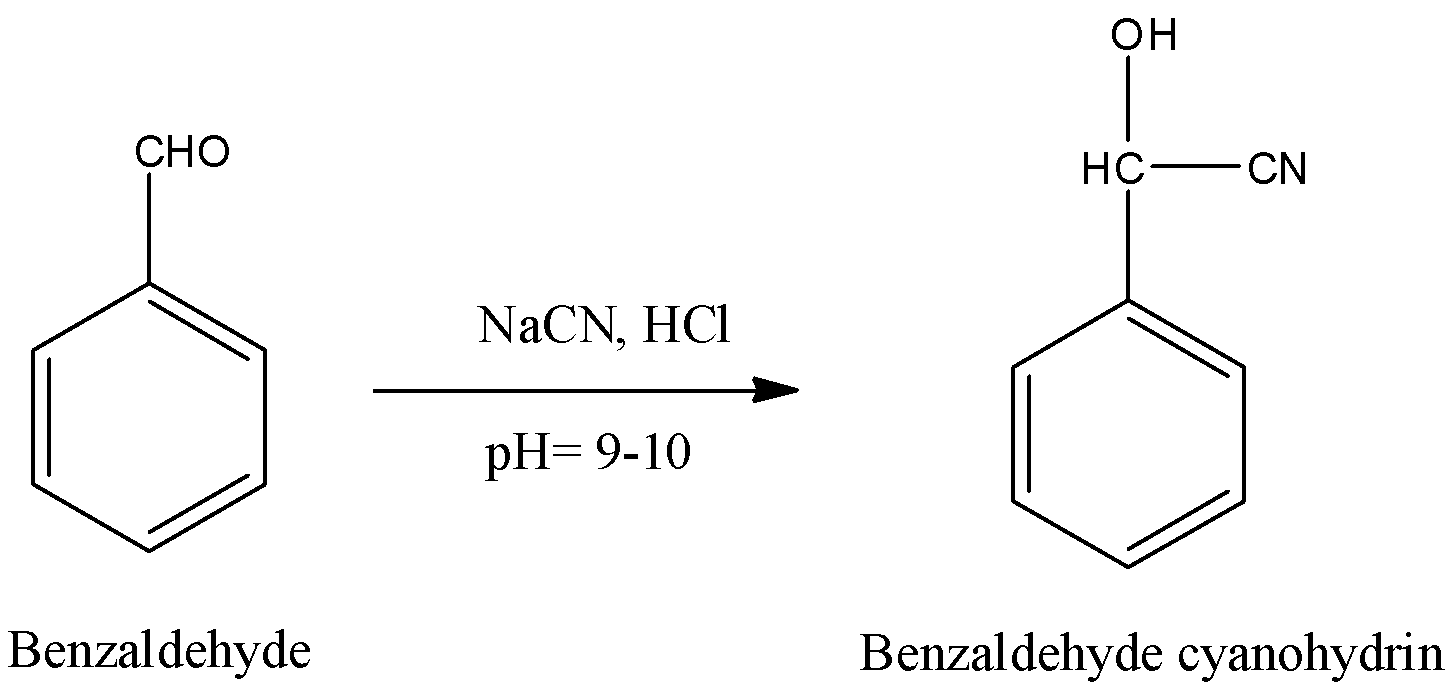
-In the above reaction Benzaldehyde undergoes bi molecular nucleophilic addition reaction with sodium cyanide to form benzaldehyde cyanohydrin as a product in step-1.
-The mechanism of nucleophilic addition reaction is as follows.
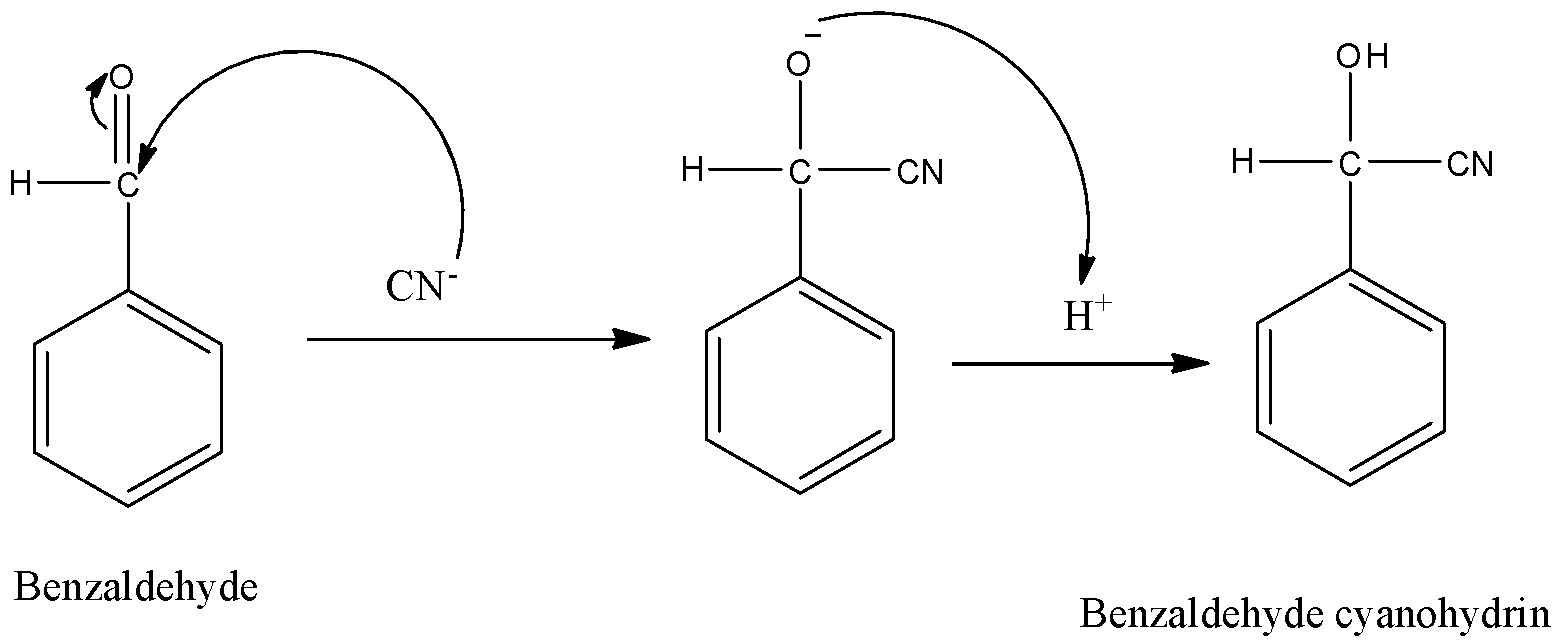
-Benzaldehyde cyanohydrin is also called as mandelonitrile.
Step-2:
-The formed benzaldehyde cyanohydrin undergoes hydrolysis in presence of acid and gives α-hydroxyphenylacetic acid. Conversion of benzaldehyde cyanohydrin to α-hydroxyphenylacetic acid is as follows.
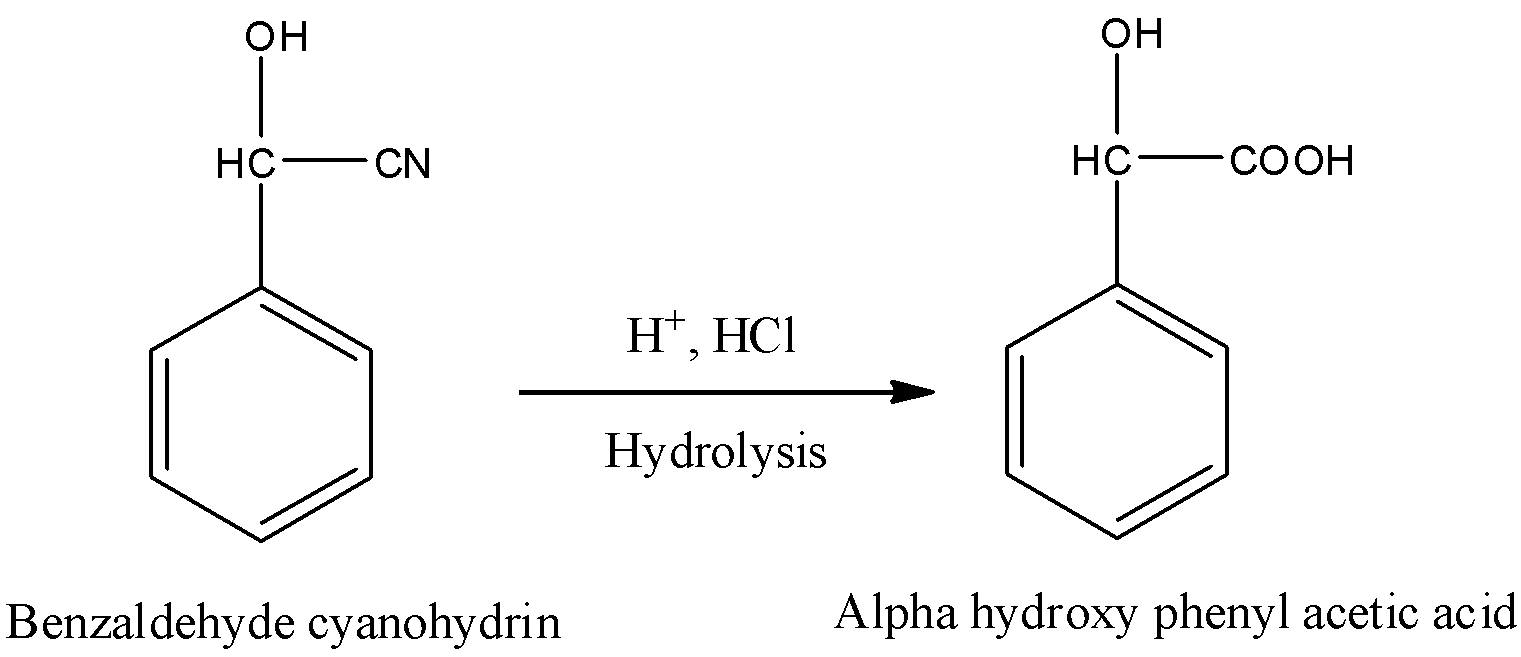
-In the above reaction cyanide undergoes hydrolysis in presence of acid and converts in to carboxylic acid in step-2.
-The mechanism of hydrolysis of cyanide is as follows. 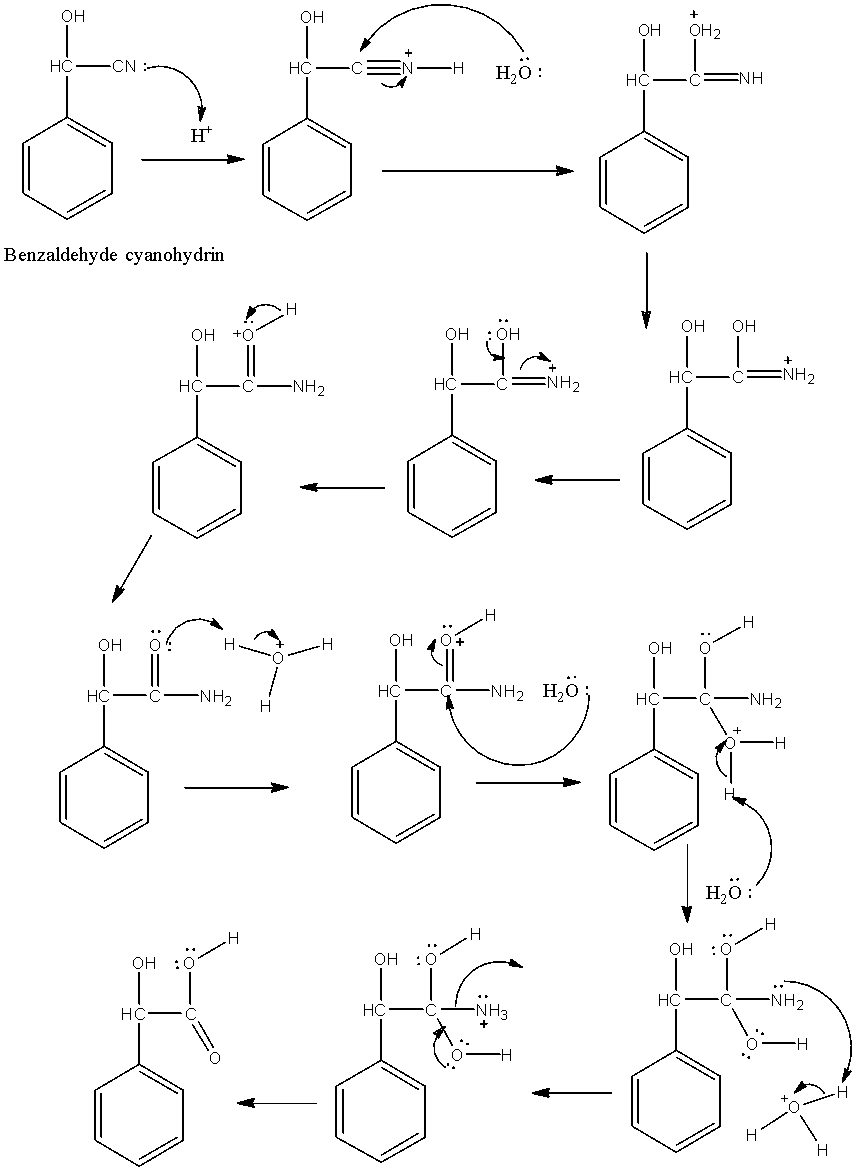
Additional information:
-α-hydroxyphenylacetic acid is also called as Mandelic acid and it is white crystalline solid.
-Mandelic acid is partially soluble in water due to the presence of carboxylic acid (-COOH) and hydroxyl (-OH) functional groups.
-Mandelic acid is completely soluble in organic solvents.
-Mandelic acid is used as a precursor in the preparation of drugs.
-Mandelic acid is used to treat urinary tract infections.
-Mandelic acid acts as an antibacterial agent.
Note: IUPAC name ofα-hydroxyphenylacetic acid is 2-hydroxy-2phenylacetic acid. α-hydroxyphenylacetic acid has one chiral center in its structure. Generally α-hydroxy acids (AHA) are used in cosmetics. Mandelic acid has two enantiomers called D- and L-Mandelic acid.
