Question
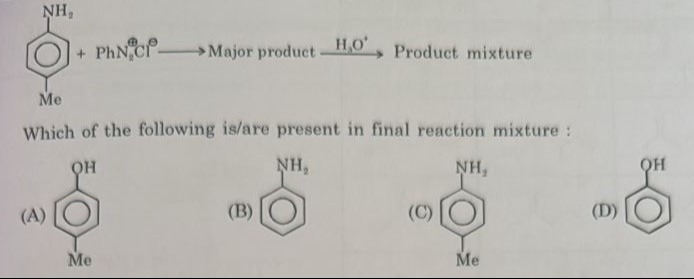
Question: $\begin{array}{c} \text{NH}_2\\ \includegraphics[width=1.0in]{image.png}\\ \text{Me} \end{array}$ + ...
NH2\includegraphicsMe
- PhN2+Cl− ⟶ Major product H2O+ Product mixture
Which of the following is/are present in final reaction mixture:
4-methylphenol
Aniline
4-methylaniline
Phenol
4-methylaniline, Phenol
Solution
The reaction sequence involves two steps. The first step is the azo coupling reaction between 4-methylaniline and benzenediazonium chloride. 4-methylaniline is an activated aromatic amine, and benzenediazonium chloride is an electrophilic diazonium salt. The coupling occurs via electrophilic aromatic substitution. The amino group is a strong activating and ortho, para-directing group. The methyl group is a weak activating and ortho, para-directing group. In 4-methylaniline, the para position to the amino group is occupied by the methyl group. Therefore, the electrophilic attack by the benzenediazonium cation occurs at the ortho position to the amino group, which is position 2 or 6. The major product is 2-(phenylazo)-4-methylaniline.
The second step is the treatment of this major product with H2O+. Azo compounds are generally stable under acidic conditions. However, if the question implies harsh acidic conditions or elevated temperature, decomposition might occur. Given the options, it is likely that some decomposition or side reaction is expected.
Let's consider the possibility that the azo coupling reaction is reversible under acidic conditions, or that the azo compound undergoes some decomposition.
Let's consider the fate of the starting materials under acidic conditions. Benzenediazonium chloride decomposes in water, especially upon heating, to form phenol, nitrogen, and HCl. 4-methylaniline is a stable amine.
Let's assume that the azo coupling is not 100% complete, and there is residual benzenediazonium chloride. Then, upon treatment with water, the residual benzenediazonium chloride decomposes to phenol. Also, if the reaction is not complete, some 4-methylaniline will be present.
Given the options, it is most likely that the question is implying incomplete reaction in the first step and subsequent decomposition of unreacted benzenediazonium chloride. Therefore, 4-methylaniline (starting material) and phenol (decomposition product of unreacted benzenediazonium chloride) are likely present in the final mixture.
