Question
Question: $\begin{array}{c} CH_2-OH \\ \mid \\ CH-OH \\ \mid \\ CH_2-OH \end{array}$ + "x" $HIO_4$...
CH2−OH∣CH−OH∣CH2−OH + "x" HIO4
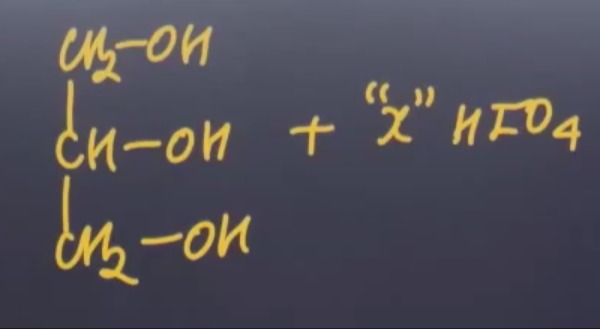
For glycerol + 2 mol HIO₄, the complete oxidation yields 3 mol of formic acid per mole of glycerol.
Solution
Solution:
We have the molecule
CH2OH∣CH(OH)∣CH2OHwhich is glycerol [HOCH₂–CHOH–CH₂OH]. Periodic acid (HIO₄) cleaves vicinal diols. In glycerol two 1,2-diol units are available. Under oxidizing acidic conditions any aldehyde formed (here formaldehyde) is further oxidized to formic acid. Overall, when glycerol is completely oxidized by periodic acid, each of its three carbons is converted into a carboxylic acid group.
The stoichiometry of the oxidation is such that 1 mole of glycerol requires 2 moles of HIO₄ to give 3 moles of formic acid:
HOCH2–CHOH–CH2OH+2HIO4⟶3HCOOH(plus iodine‐containing by‐products)Minimal Explanation:
-
Glycerol has two vicinal diol segments.
-
HIO₄ oxidatively cleaves these diols.
-
Cleavage yields carbonyl fragments; under acid conditions, formaldehyde is further oxidized to formic acid.
-
Overall, every carbon becomes an acid group; thus, 1 mole glycerol gives 3 moles formic acid with 2 moles HIO₄.
Answer:
For glycerol + 2 mol HIO₄, the complete oxidation yields 3 mol of formic acid per mole of glycerol.
