Question
Question: \(Be\) in \(BeC{l_2}\) undergoes : A) Linear hybridisation B) Trigonal hybridisation C) Tetra...
Be in BeCl2 undergoes :
A) Linear hybridisation
B) Trigonal hybridisation
C) Tetrahedral hybridisation
D) No hybridisation
Solution
First we should be aware of the molecule of the given chloride. Now after that we can say that it has a specific geometry as the bonds formed are covalent and have a special and unique integrity of banana bonding as a whole. But when it comes to the molecule individually it is linear in nature.
Complete step by step answer:
Here in the given question statement the question is regarding the hybridisation of the individual atoms in the chloride of beryllium. Now we first have to know what exactly chloride is. The formula is already given, so we can get the brief idea and then we can make the structure of the molecule and then we can get the answer.
So let's do the answer step by step.
Beryllium chloride is the name given to the chloride. It is an inorganic compound. Apart from that we can say that it is a colourless compound. It is also a hygroscopic solid in physical aspect as that dissolves well in many polar solvents.
Now talking about the structure we get that in the molecule of BeCl2 , the central atom of Be is in sp hybridization. Now this type of hybridisation is a linear hybridisation.
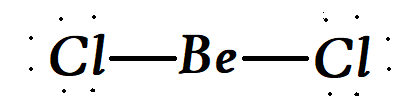
Therefore we can say that the right option is option A,Linear hybridisation.
Note: The solid is a 1-dimensional polymer consisting of edge-shared tetrahedra. In contrast, BeF2 is a 3-dimensional polymer, with a structure akin to that of quartz. In the gas phase, BeCl2 exists both as a linear monomer and a bridged dimer with two bridging chlorine atoms where the beryllium atom is 3-coordinate.
