Question
Question: Based on the group valency of elements write the molecular formula of the following complex followin...
Based on the group valency of elements write the molecular formula of the following complex following compounds giving justification for each:
(i) Oxide of first group elements.
(ii) Halides of the elements of group thirteen.
(iii) Compound formed when an element A of a group 2 combines with an element B of group seventeen.
Solution
We know that valence electrons are defined as the number of electrons present in the outermost shell and the number of electrons it accepts or donates to form a bond is called the valency of an electron.
For Example, The number of valence electrons in carbon is four and it needs four more electrons to have filled the outermost electron. Thus the valency of carbon is four.
Complete answer:
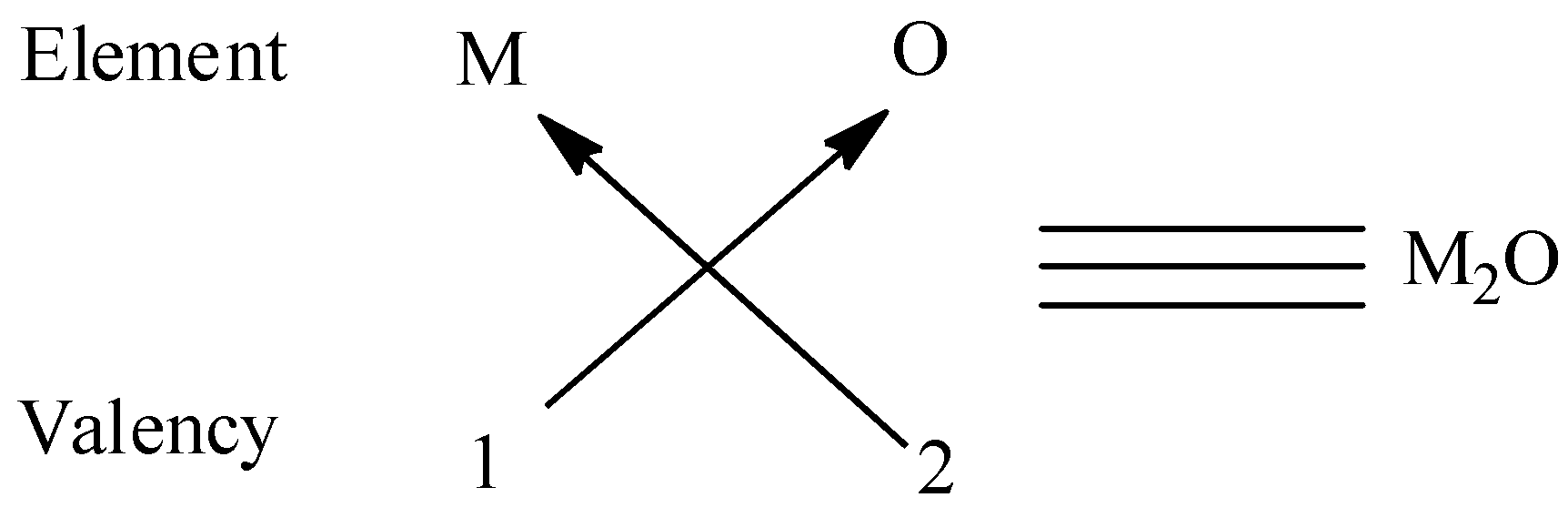
(i) We have to know that the valency of first group elements is 1 and the valency of oxygen is 2 and to satisfy the combining capacity of oxygen, 2 elements of first group are required. Thus the oxide of the first group elements has the common formula of M2O. We can give an example for this type is Na2O .
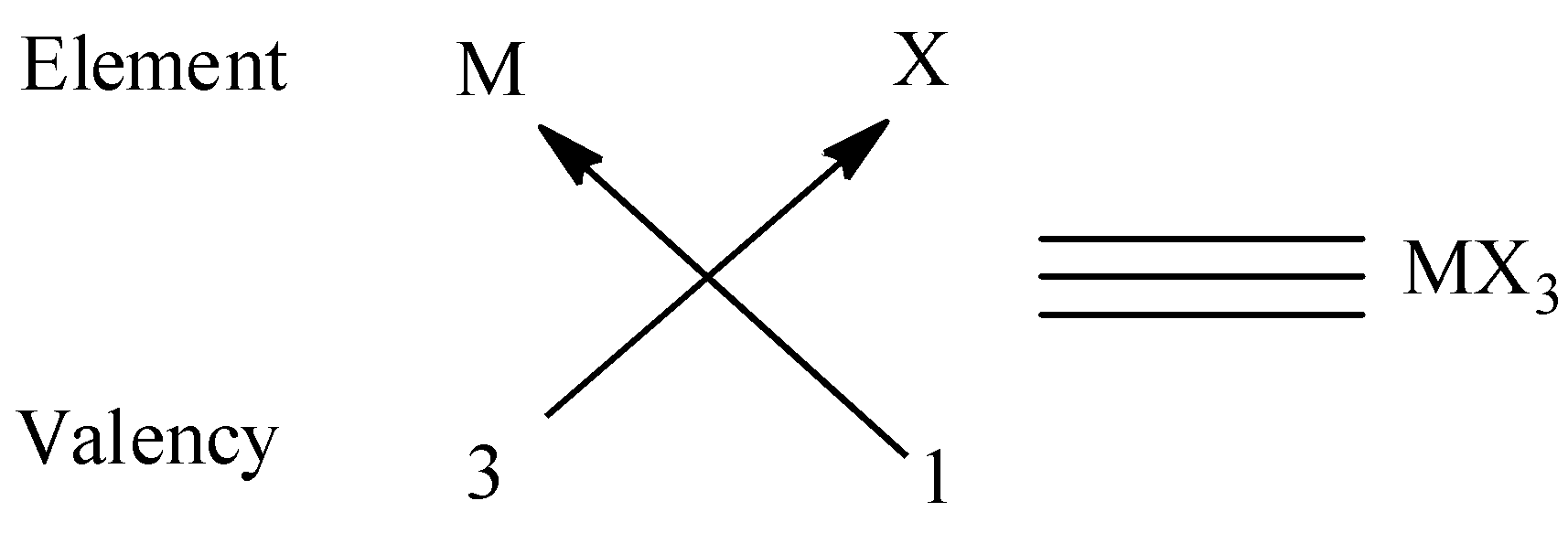
(ii) We know that the valency of group 13 elements is 1 and the valency of oxygen is2 and to satisfy the combining capacity of group 13 elements, 3 halogens are required. Thus the molecular formula form is MX3. We can give an example for this type is BCl3 .
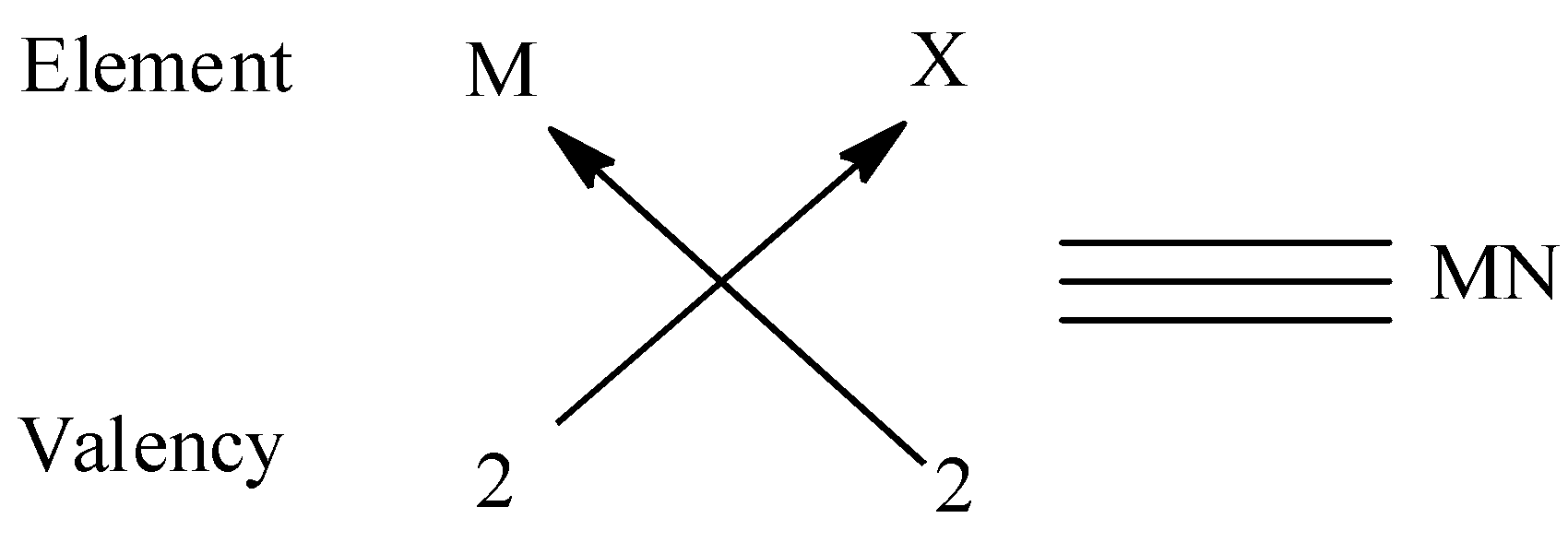
(iii) We must remember that the valency of group 2 elements is 2 and that of group 17 is 1 . Thus two elements of group 17 are required to combine with group 2 elements. Thus the molecular formula form is MN. We can give an example for this type is MgO.
Note:
We must remember that some elements vary in their capability to react with other elements, depending on the nature of the reaction; variable valence. For example, iron (Fe) may have a valence of both 2 & 3.
