Question
Question: Based on the energy diagram, what type of reaction could this represent? 
(A) The reaction is an endothermic, two-step reaction that involves the formation of an intermediate.
(B) The reaction is an exothermic, two-step reaction that involves the formation of an intermediate.
(C) The reaction is an endothermic reaction that involves an activated complex.
(D) The reaction is an exothermic reaction that involves an activated complex.
Solution
Energy diagram can be used to represent the change in potential energies of reactants, products, or even formation of the transitional state with respect to time. If energy increases over time, then it means energy is being absorbed by the compounds.
Complete step-by-step answer
Whenever a reactant is converted into the product by either absorption or release of energy it is known as a chemical reaction. It can be of two types, Endothermic reaction or exothermic reaction.
The exothermic reaction involves the release of energy in the form of either heat, light, etc. when reactants are converted into products.
Endothermic reactions involve the formation of products from reactants by absorption of heat energy from the surrounding, making the surrounding cool. In an endothermic reaction the potential energy of reactant is lower compared to the product that is reactant absorbs energy from the surrounding to convert into the product, therefore the energy diagram indicates a rising curve.
In the above diagram,
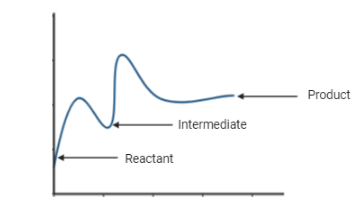
Here, as we can see, the potential energy of the reactant is lower compared to the product; this reaction is a two-step endothermic reaction with the formation of one intermediate.
Hence, the correct option will be option A - The reaction is an endothermic, two-step reaction that involves the formation of an intermediate.
Note:
In a system, whenever a chemical bond is formed in the reaction energy is required similarly breaking of bond involves the release of energy. An endothermic reaction is associated with the formation of a bond where the enthalpy of the system is positive, whereas in exothermic reaction energy is released hence, the enthalpy of the system is always negative.
