Question
Question: Balance the following redox reactions: \({I_2}(s) + OC{l^ - }(aq) \to IO_3^ - (aq) + C{l^ - }(aq)\...
Balance the following redox reactions:
I2(s)+OCl−(aq)→IO3−(aq)+Cl−(aq)
(A) I2(s)+5OCl−(aq)+H2O(l)→2IO3−(aq)+5Cl−(aq)+2H+(aq)
(B) I2(s)+5OCl−(aq)+2H2O(l)→IO3−(aq)+4Cl−(aq)+2H+(aq)
(C) I2(s)+3OCl−(aq)+4H2O(l)→IO3−(aq)+4Cl−(aq)+2H+(aq)
(D) I2(s)+5OCl−(aq)+7H2O(l)→IO3−(aq)+5Cl−(aq)+2H+(aq)
Solution
Redox reaction is a reaction in which oxidation and reduction takes place simultaneously.
Oxidation is a process in which the oxidation number of atoms increases and Reduction is a process in which the oxidation number of atoms decreases.
Therefore, the basic principle of the reaction is that during redox reaction total increase in oxidation number must be equal to total decrease in oxidation number.
Complete step by step answer:
The chemical equation is:
I2(s)+OCl−(aq)→IO3−(aq)+Cl−(aq)
Let us find the oxidation number of each element in the above reaction.
(i) Since I2(s) is present in an elemental state therefore its oxidation number is zero.
(ii) Oxidation number of Cl in OCl−: I2(s)
-2 + x = - 1(because total charge on anion is -1)
x = - 1 + 2
x = + 1
(iii) Oxidation number of I in IO3−(aq):
IxO3−−2=x+(−2×3)
x - 6 = - 1(because total charge on anion is -1)
x = - 1 + 6
x = + 5
(iv) Oxidation number of Cl− is - 1.
The complete redox reaction is given below:
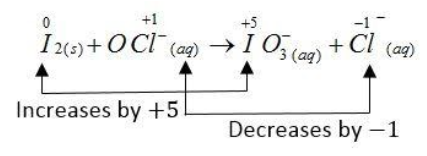
The oxidation number of the I atom increases from 0 to + 5 and the oxidation number of Cl atom decreases from + 1 to - 1.
I2(s)+OCl−(aq)→IO3−(aq)+Cl−(aq)
Now balance the O-atom by adding a number of water molecules to the side which is deficient in O-atom.
I2(s)+5OCl−(aq)+H2O(l)→2IO3−(aq)+5Cl−(aq)
Now balance the hydrogen atom by adding H+ to the deficient side.
I2(s)+5OCl−(aq)+H2O(l)→2IO3−(aq)+5Cl−(aq)+2H+(aq)
This is the balanced equation.
Therefore, from the above explanation the correct option is (A).
Note: Balancing of Hydrogen atoms depend upon the medium acidic or basic.
If reaction takes place in an acidic medium then H+ ion is added to the side deficient in Hydrogen atom.
If reaction takes place in the basic medium add H2O molecule to the H-atom deficient side and simultaneously add OH− on the other side.
