Question
Question: Balance the following equation by oxidation number method. \( {K_2}C{r_2}{O_7} + HCl \to KCl + Cr...
Balance the following equation by oxidation number method.
K2Cr2O7+HCl→KCl+CrCl3+H2O+Cl2
Solution
Hint : The oxidation state or sometimes known as oxidation number, defines the degree of oxidation that an atom possesses in a chemical compound. In other words, oxidation number refers to the charge left on the central atom especially when all the bonding pairs of electrons get broken, with the charge allocated to the most electronegative atom.
Complete Step By Step Answer:
Oxidation number method is generally used to balance the redox reactions. This method works on the principle that the amount of oxidation equals the amount of reduction in the whole chemical reaction. Now using this method, we will balance the given chemical equation stepwise as stated below:
Step 1: To write down the correct molecular formula for each of the reactants and products.
K2Cr2O7+HCl→KCl+CrCl3+H2O+Cl2
In the given chemical equation, all molecular formula provided is correct.
Step 2: To identify the elements which change their oxidation number during the execution of reaction. We will write the oxidation numbers of atoms above their chemical symbols on each side of the chemical equation.
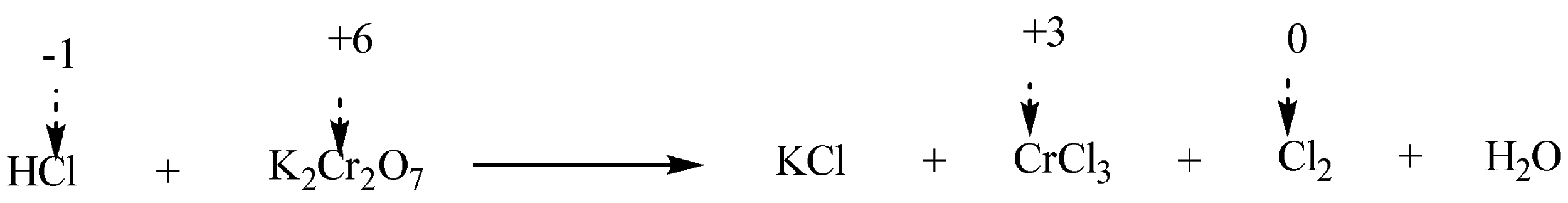
It can be thus visualised that Cl ion is getting oxidized while Cr ion is getting reduced.
Step 3: To find out the increase or decrease in oxidation number of each atom. Make them equal by multiplying with a suitable number.
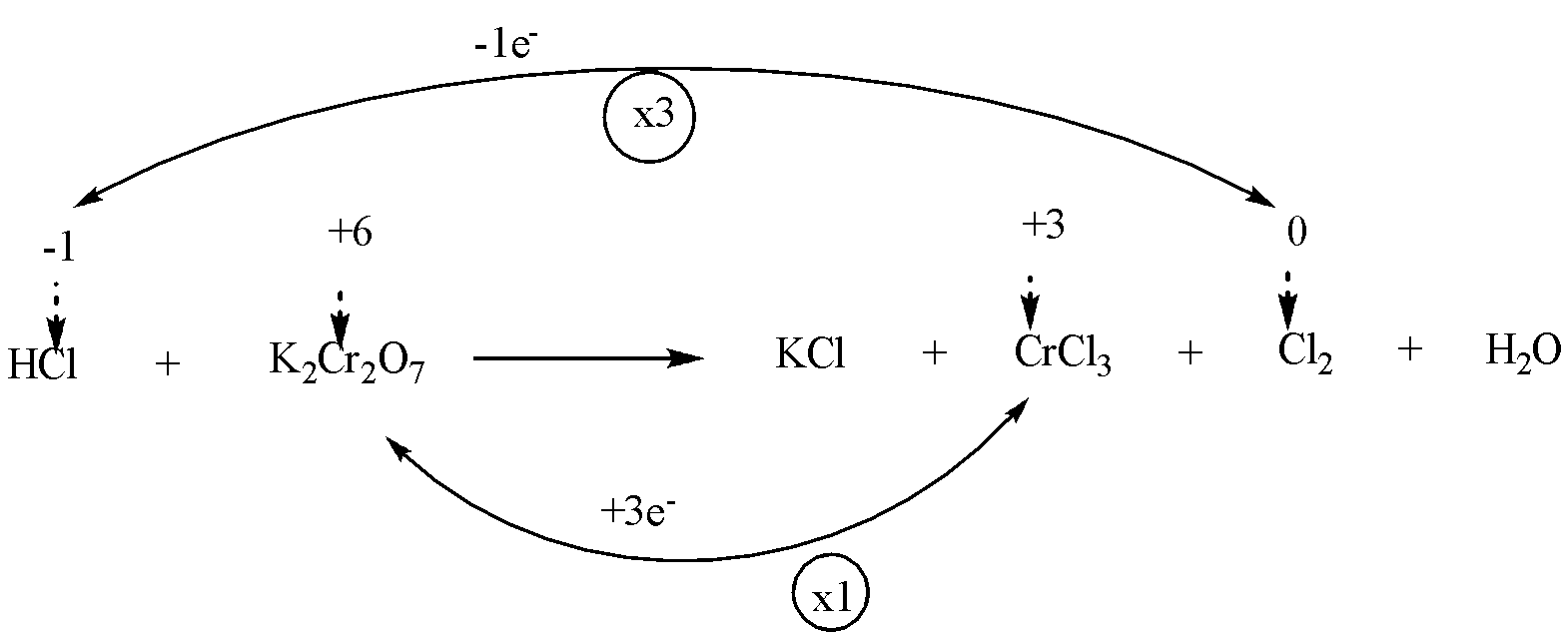
We will multiply HCl and Cl2 by 3 and K2Cr2O7 and Cl2 by 1.
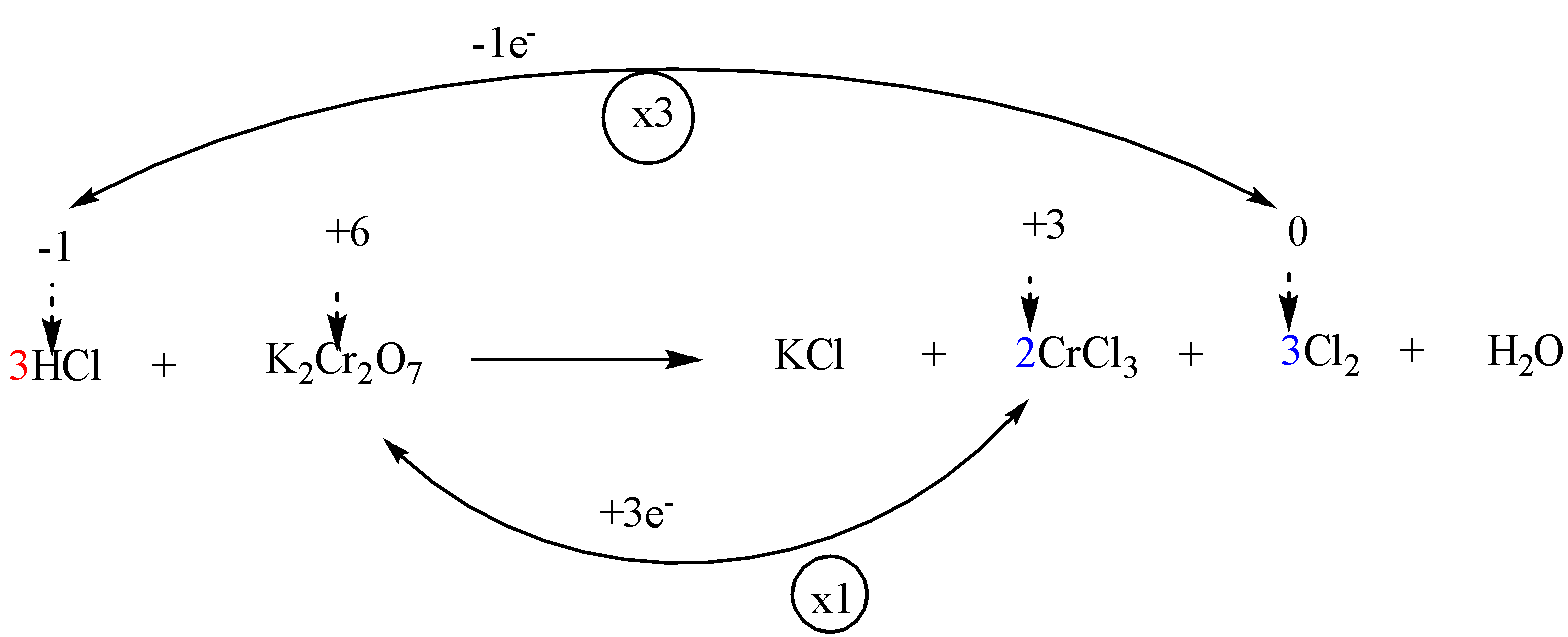
Step 4: To balance the metal ions which don’t change their oxidation number.
Step 5: Add H+ (in case of acidic solutions) or OH− ions (in case of alkaline solutions) to the chemical reaction on appropriate sides such that total ionic charges of the reactants as well as products are same.
The given reaction in question is in acidic solution (i.e. HCl ). Thus, HCl must be added on the side of reactants in order to balance 14 Cl atoms on the side of products. Therefore, the coefficient of HCl will be 14.
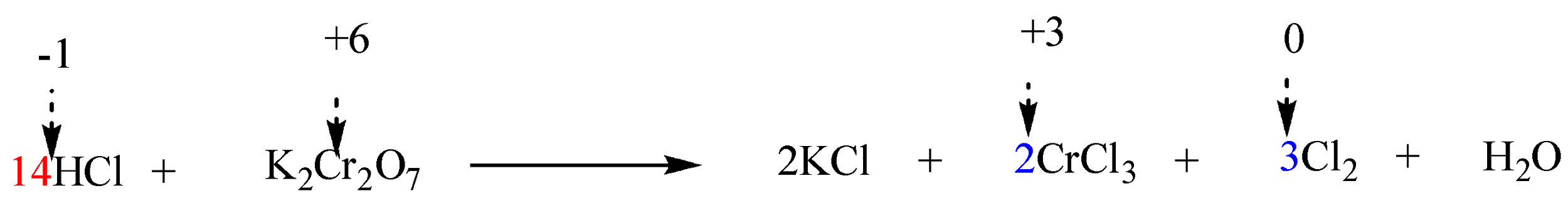
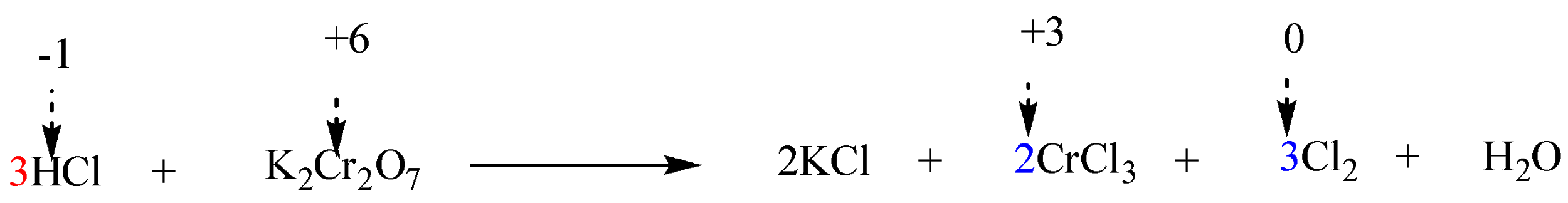
Step 6: Finally balance the number of hydrogen and oxygen atoms in the expression on each side by adding H2O molecules.
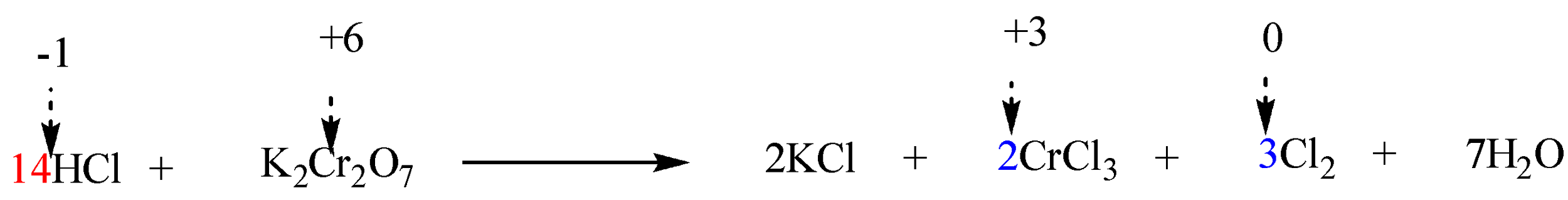
The reaction is now completely balanced.
Note :
A balanced chemical equation simply obeys the law of conservation of mass. Balancing the chemical equations is a significant guiding principle in chemistry. A balanced chemical equation helps you to predict the amount of reactants required and the amount of products formed.
