Question
Question: Among the following, the conformation that corresponds to the most stable conformation of meso-butan...
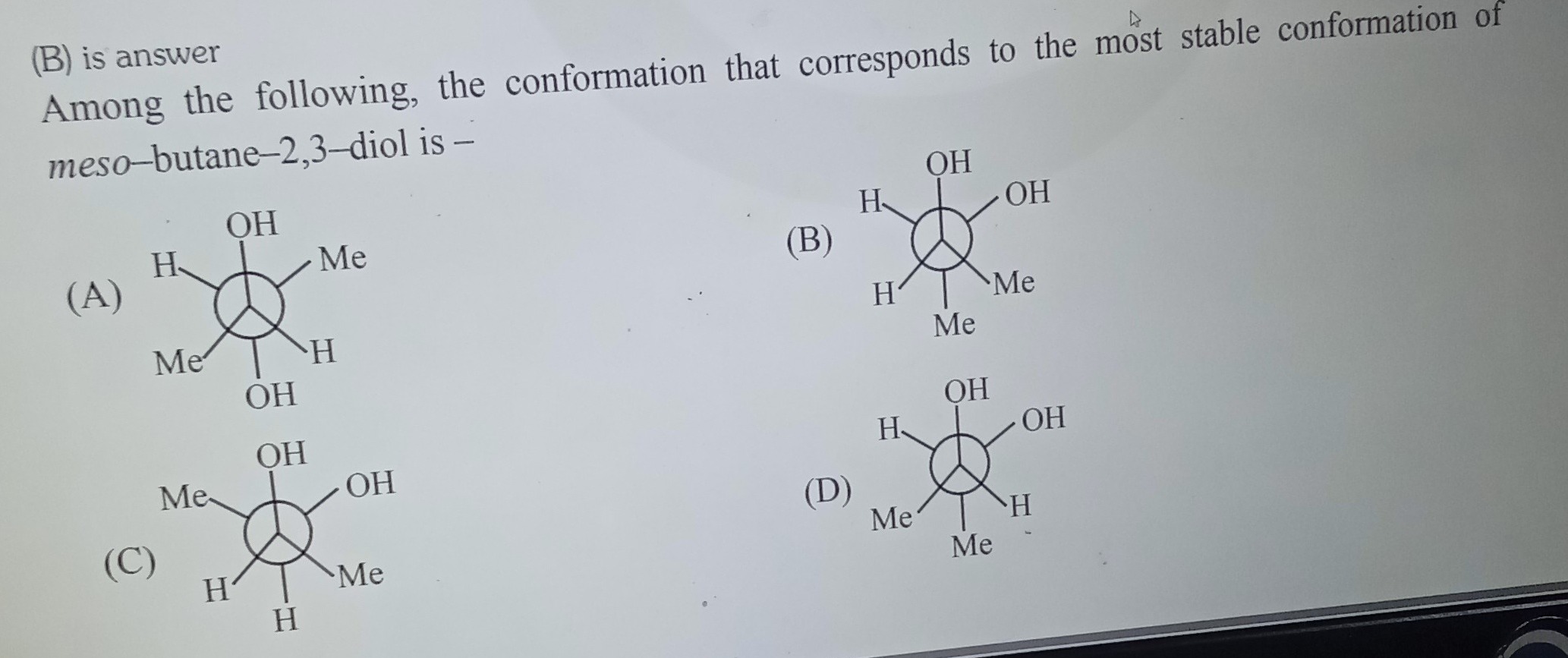
Among the following, the conformation that corresponds to the most stable conformation of meso-butane-2,3-diol is -
B
Solution
The molecule is meso-butane-2,3-diol, meaning it has two chiral centers with opposite configurations (R and S) and possesses an internal plane of symmetry. We're looking for the most stable conformation among the given Newman projections.
The stability of conformations is determined by torsional strain and steric strain. Staggered conformations are more stable than eclipsed conformations. Among staggered conformations, the anti conformation of bulky groups is generally the most stable due to minimized steric repulsion. However, in molecules with polar groups like OH, intramolecular hydrogen bonding can occur, stabilizing a gauche conformation where the OH groups are close (dihedral angle approximately 60 degrees).
Let's analyze the options:
-
(A) Staggered. OH is anti to OH, Me is anti to Me, H is anti to H. All bulky groups are anti to each other, minimizing steric repulsion.
-
(B) Staggered. OH is gauche to OH (dihedral angle approximately 60 degrees), Me is anti to Me. The gauche interaction between OH groups can be stabilized by intramolecular hydrogen bonding.
-
(C) Eclipsed. This conformation has maximum torsional strain and steric repulsion due to eclipsed groups. It is the least stable.
-
(D) Staggered. OH is anti to OH, Me is gauche to Me. The gauche interaction between the two Me groups leads to significant steric repulsion.
Comparing the staggered conformations (A), (B), and (D), we consider the balance between steric repulsion and intramolecular hydrogen bonding. Intramolecular hydrogen bonding in 1,2-diols is known to stabilize the gauche conformation where the OH groups are gauche to each other. This stabilization can outweigh the steric repulsion of the gauche interaction between the OH groups.
Therefore, the most stable conformation is the one where the bulky Me groups are anti and the OH groups are gauche, allowing for intramolecular hydrogen bonding. This corresponds to option (B).
