Question
Question: (B) In the Below Reaction is-  In the Below Reaction is-
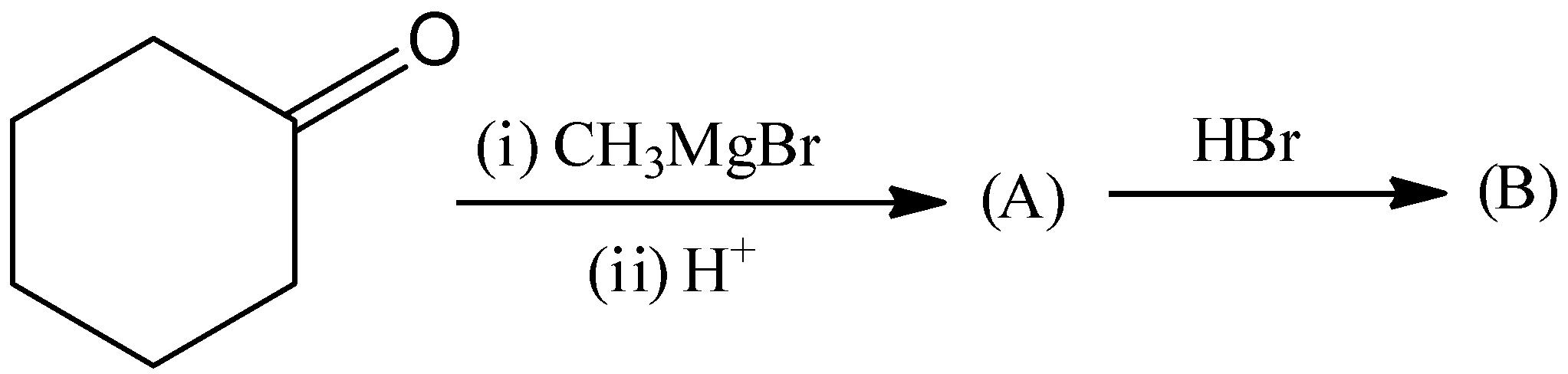
A.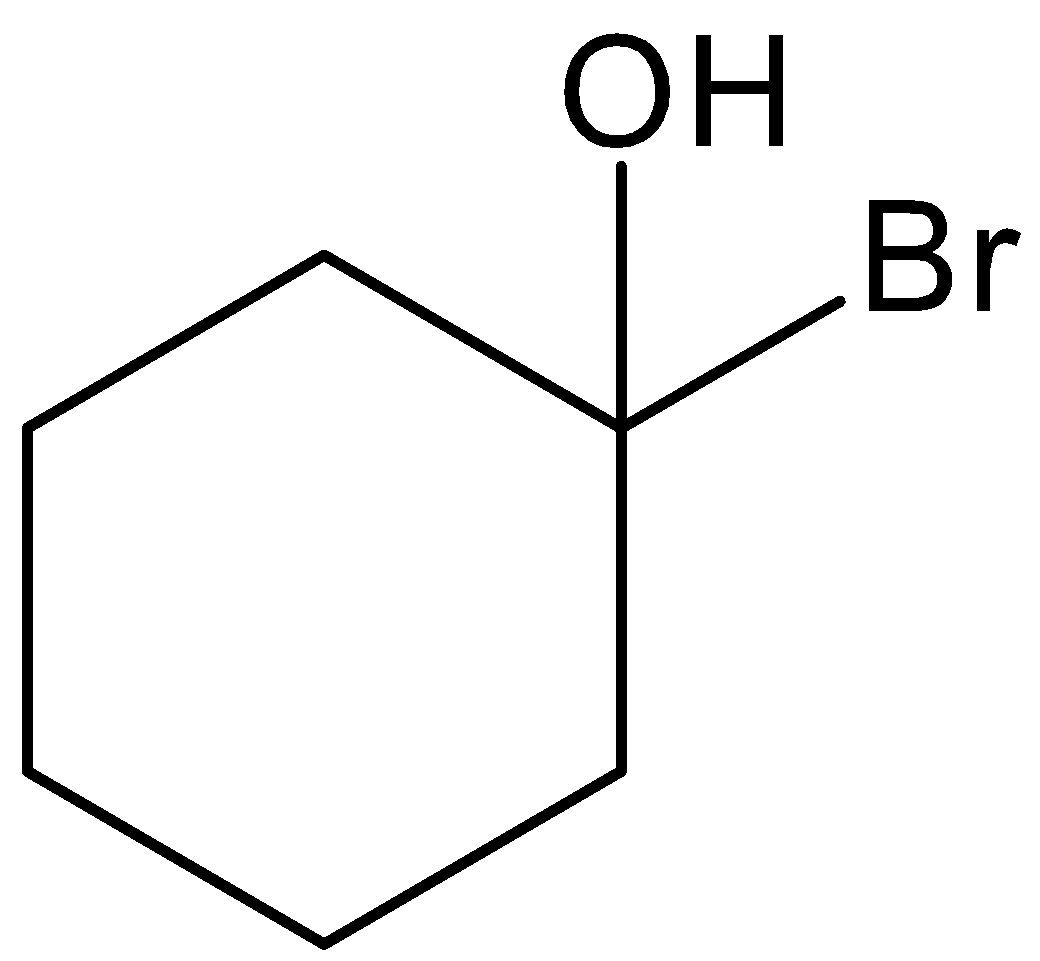
B.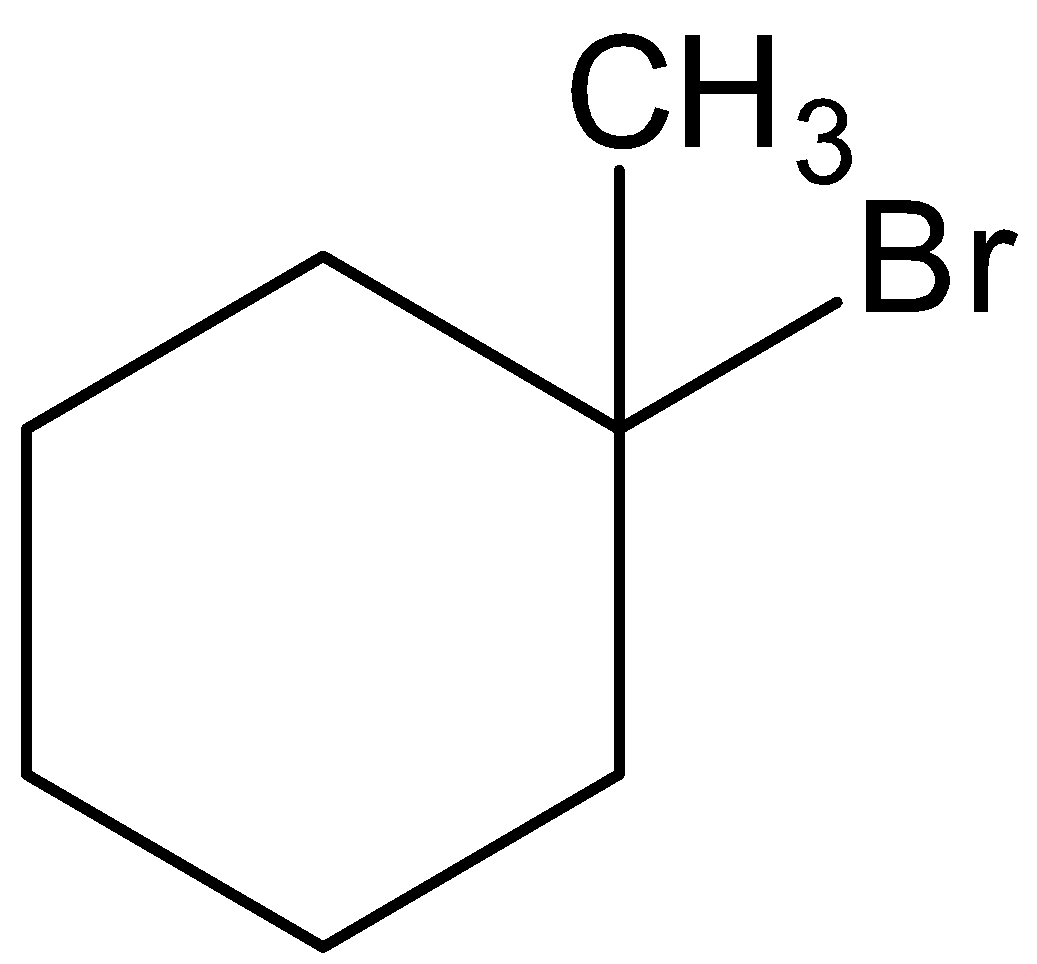
C.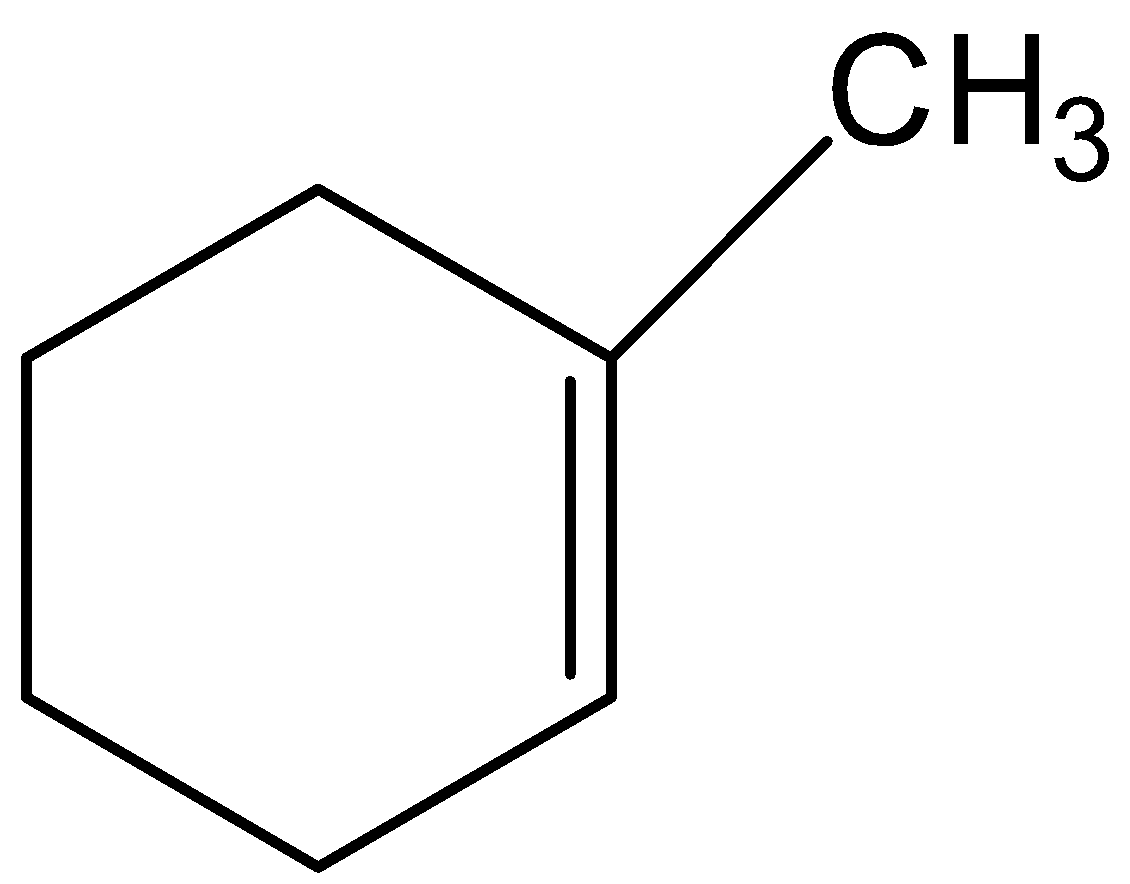
D.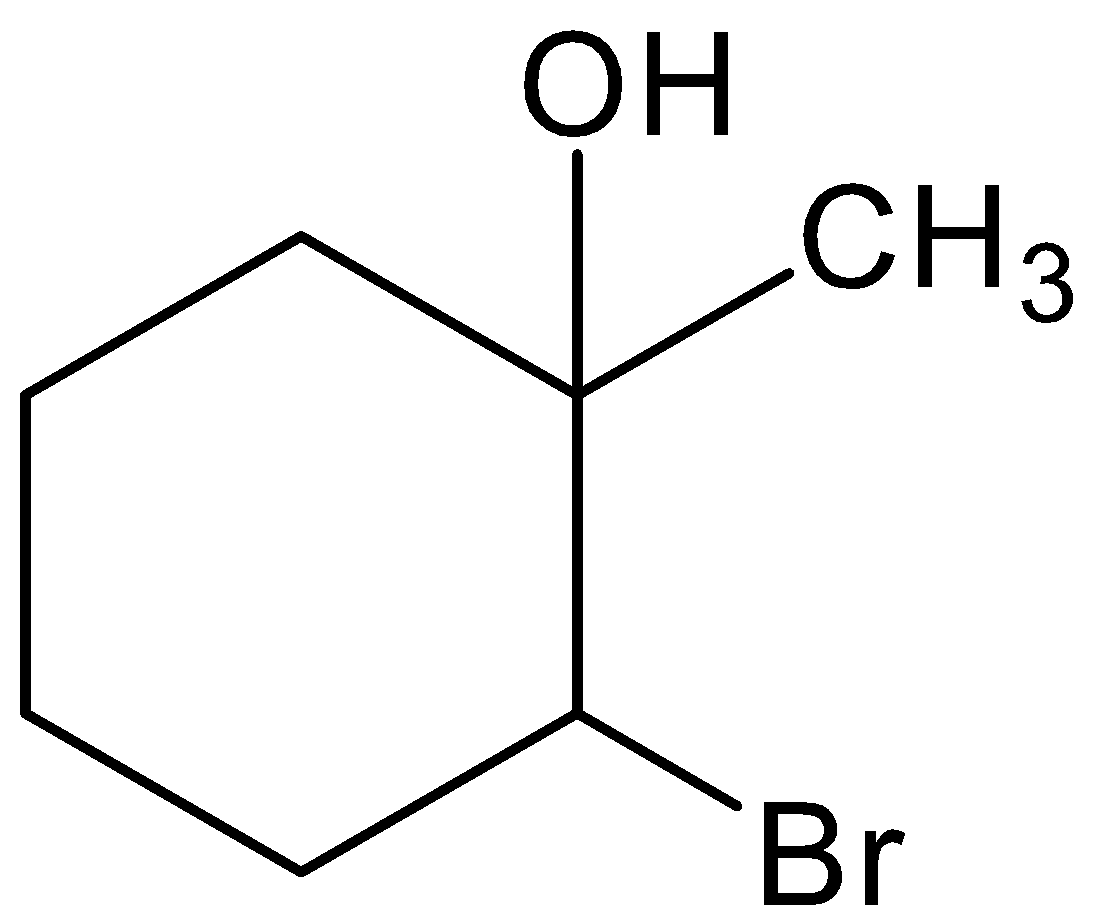
Solution
We must have to know that the cyclohexanone is a chemical compound having the formula, (CH2)5CO. The cyclohexanone is a chemical compound with a functional group, which is ketone. And this is mainly used in paints, acetate cellulose, polymers, copolymers, etc as a solvent. The cyclohexanone is dangerous to our health. Because, it causes headaches, dizziness etc.
Complete answer:
This is not the correct structure of compound (B). Hence, option (A) is incorrect.
Here, the cyclohexanone is reacted with methyl magnesium bromide, (Grignard reagent) and it undergoes hydrolysis and there is a formation of 1−methylcyclohexan−1−ol. Let’s see the reaction,
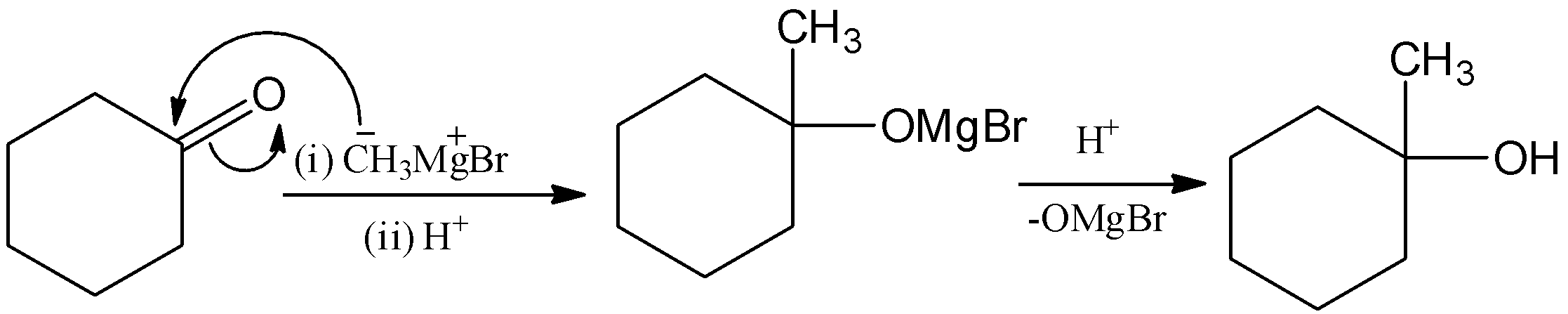
Here, the methyl group will attack the carbon group and form magnesium 1−methylcyclohexan−1−olate bromide. And it undergoes hydrolysis, then –OMgBr is replaced by –OH group. Hence, there is a formation of1−methylcyclohexan−1−ol.
This 1−methylcyclohexan−1−ol is reacted with hydrogen bromide and there is a formation of 1-bromo-1-methylcyclohexane. Let’s see the reaction with mechanism,
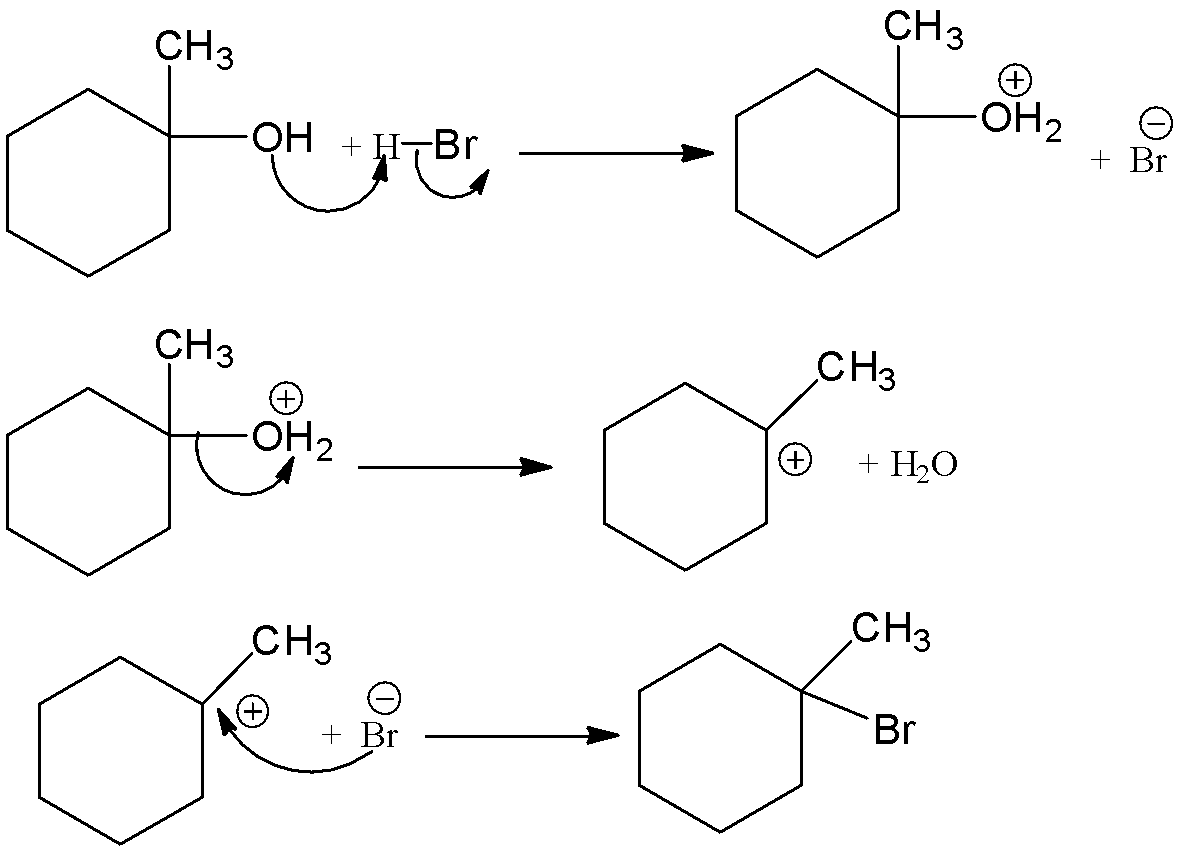
Thus, overall the reaction can be written as,
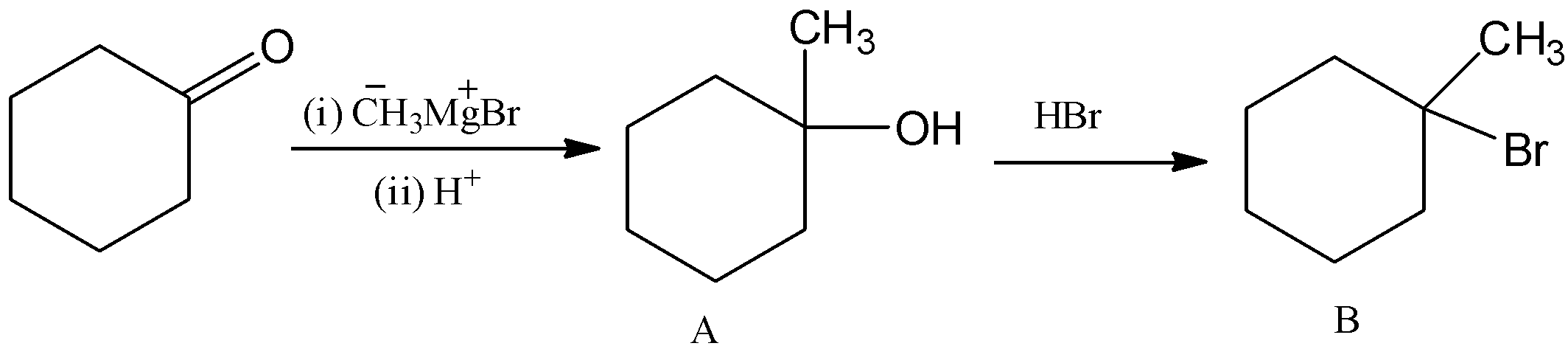
Hence, option (B) is correct.
This is not the correct structure of B. option (C) is incorrect.
The given compound is not the correct structure of compound B. Hence, option (B) is incorrect.
Hence, the option (B) is correct.
Note:
Here, the cyclohexanone is reacted with Grignard reagent and it undergoes the hybridization reaction. And the Grignard reagent acts as nucleophiles. Here, the cyclohexanone is a ketone. And when the ketone is reacted with Grignard reagent, the carbonyl group is converted into alcohol. This alcohol is treated with hydrogen bromide, and it undergoes the nucleophilic substitution reaction.
