Question
Question: B−H−B bridge in \[{{B}_{2}}{{H}_{6}}\]is formed by sharing of: (A) 2 electrons (B) 4 electrons ...
B−H−B bridge in B2H6is formed by sharing of:
(A) 2 electrons
(B) 4 electrons
(C) 1 electrons
(D) 3 electrons
Solution
Hint: To answer this question, we should first draw the structure of diborane. When we draw the structure of a diborane, then only we can tell the number of electrons in the BHB bridge.
Complete step by step solution:
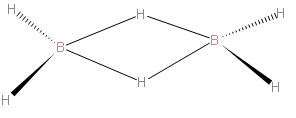
We should first know about diborane. It has the chemical formula of B2H6. - It is the chemical compound consisting of boron and hydrogen with the formula B2H6. So first we will draw the structure of Diborane. We should carefully draw the structure of Diborane. We should know that four hydrogen in diborane are attached to terminals, while two hydrogens act as bridges between the boron centers. We should understand that the lengths of the B-H bridge bonds and the B-H terminal bonds are 1.33 and 1.19 Å, respectively. This difference in bond lengths reflects the difference in their strengths, the B-H bridge bonds being relatively weaker.
The above structure represented is of B2H6..
As we draw the structure ofB2H6, it describes the bonds between boron and the terminal hydrogen atoms as traditional 2-center, 2-electron covalent bonds. The bonding between the boron atoms and the bridging hydrogen atoms is, however, different from that in molecules such as hydrocarbons. We know that boron uses two electrons in bonding to the terminal hydrogen atoms, and has one valence electron remaining for additional bonding. The bridging hydrogen atoms provide one electron each. The B2H2 ring is held together by four electrons which form two 3-center 2-electron bonds. This type of bond is sometimes called a 'banana bond'.
So, from the above discussion, we came to know that the B-H-B bridge in B2H6 is formed by sharing two electrons. Therefore correct option is (A).
Note:We should note that diborane can be used as a rocket propellant. The complete combustion of diborane is strongly exothermic. But we should also know that this shows the similarity with carbon monoxide and doesn’t burn completely.