Question
Question: (b) <figure/>...
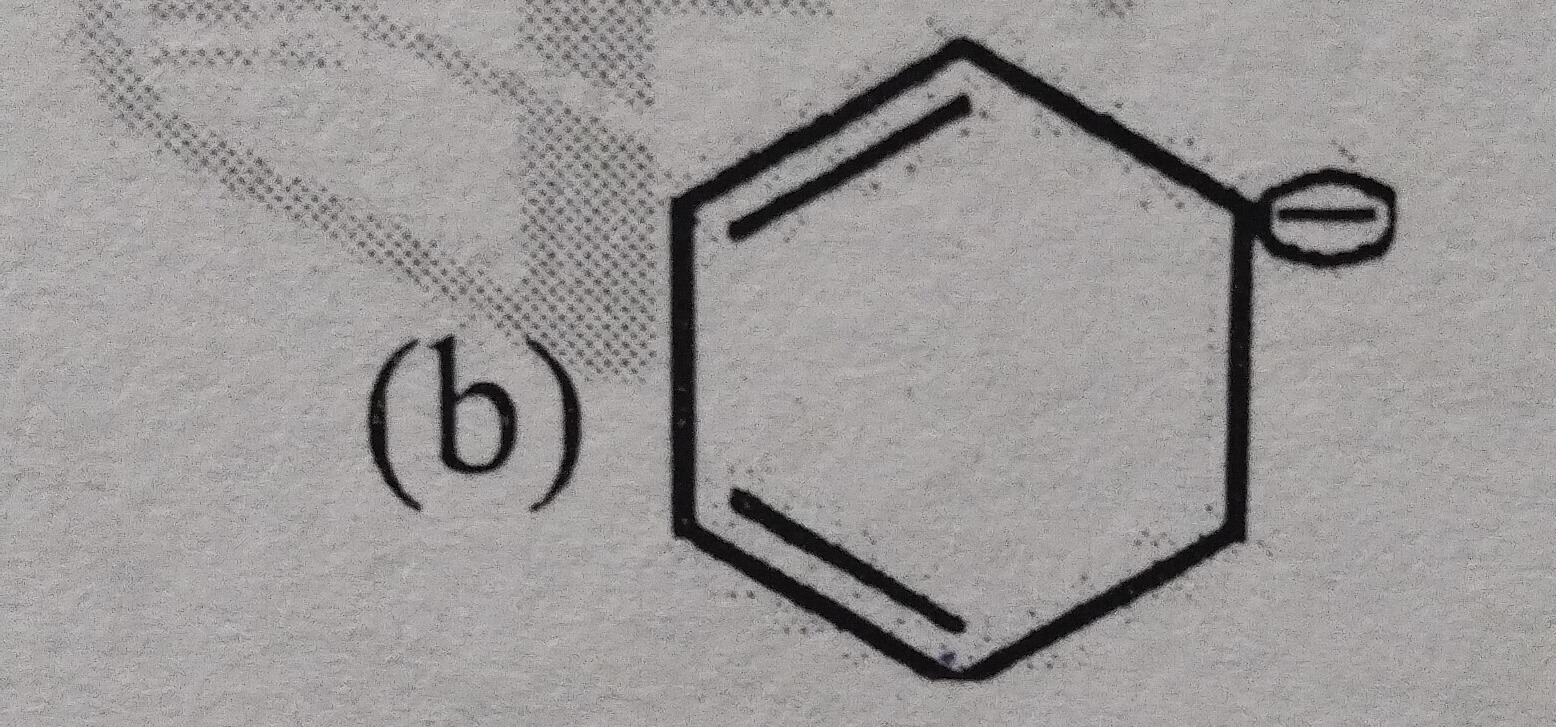
(b)
Answer
Non-aromatic
Explanation
Solution
The molecule is a 6-membered cyclic species with two double bonds and a negative charge. This structure, a cyclohexadienyl anion, contains one sp3 hybridized carbon atom within the ring. The presence of an sp3 carbon interrupts the continuous conjugation of p-orbitals around the entire ring. For a molecule to be aromatic or anti-aromatic, it must be fully conjugated. Since this condition is not met, the species is classified as non-aromatic.
