Question
Question: \(B{{F}_{3}}\) is a planar molecule whereas \(N{{F}_{3}}\) is pyramidal because: (A) B - F bond is...
BF3 is a planar molecule whereas NF3 is pyramidal because:
(A) B - F bond is more polar than N - F bond
(B) Boron atom is bigger than nitrogen atom
(C) Nitrogen is more electronegative than boron
(D) BF3 has no lone pair but NF3 has a lone pair of electrons.
Solution
Write the electronic configuration of boron and nitrogen atoms. Draw the structure of boron fluoride and nitrogen trifluoride. Take a look at the types of electron-pairs present in the molecule and then determine the correct reason.
Complete step by step solution:
- Boron has atomic number 5 and nitrogen has atomic number 7. Their electronic configuration is given as follows,
5B=1s22s22p1
7N=1s22s22p3
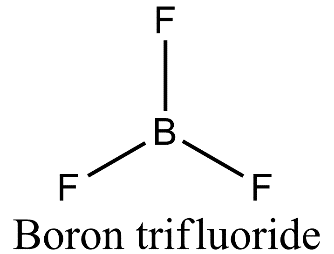
- Boron is forming three covalent bonds with fluorine so it is sp2 hybridized due to the presence of three electrons present in the outermost shell. On excitation of boron atom, the electronic configuration will be 5B=1s22s12p2 and so, three orbitals of three fluorine atoms will axially overlap with three sp2 hybridized orbitals of boron to form boron trifluoride.
- Nitrogen is also forming three covalent bonds with fluorine but when nitrogen atom is excited, the electronic configuration is 7N=1s22s12p4 and so, nitrogen undergoes sp3 hybridization in which one of the sp3 hybrid orbital will have a pair of electrons and three other orbitals will have one electron each. These three orbitals having a single electron will axially overlap with three orbitals of three fluorine atoms to form nitrogen trifluoride.
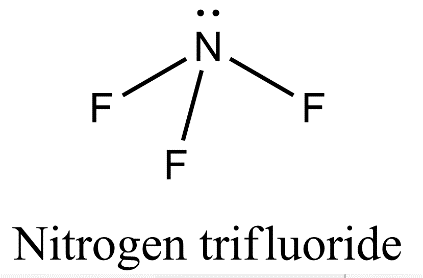
- Now, since nitrogen contains one lone pair of electrons there will be lone pair- bond pair repulsion which will lead to distortion of trigonal planar geometry. Therefore, nitrogen trifluoride will have pyramidal geometry.
- In case of boron trifluoride, there is vacant 2p orbital and no lone pair of electrons and so there is only bond pair- bond pair repulsion giving rise to trigonal planar geometry.
- Therefore, BF3 is a planar molecule whereas NF3 is pyramidal because BF3 has no lone pair but NF3 has a lone pair of electrons.
- Hence, option (D) is the correct answer.
Note: Remember lone pair - lone pair repulsion is greater than lone pair- bond pair repulsion which is greater than bond pair- bond pair repulsion. Due to the presence of lone pairs, there is distortion of geometry.
