Question
Question: Azide ion \[(N_3^ - )\] exhibits \[N-N\] bond order of 2 and maybe represented by resonance structur...
Azide ion (N3−) exhibits N−N bond order of 2 and maybe represented by resonance structures I, II and III given below.
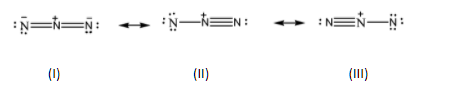
Select the correct statements.
A.Structures I and II make greater contributions than III
B.Structures II and III make smaller contributions than I
C.Structures I and III make greater contributions than II
D.All three structure make equal contributions
Solution
Let us first discuss resonance hybrid structures. In any given molecule or ion or atom which exhibits resonance, there exist a few resonance forms that can be understood as the average of two or more resonance structures that define the given species. They are represented as separate structures from the other resonance structures by using double headed arrows.
Complete Step-by-Step Answer:
Before we move forward with the solution of the given question, let us first understand some important basic concepts.
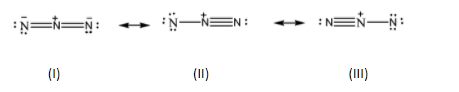
Structures II and III are almost similar structures. We can observe that the pi bond from the first nitrogen atom in structure II is shifted to the third nitrogen atom in structure III. Both these structures are formed by shifting the electron bond over the central nitrogen atom.
On the other hand, if we observe structure I closely, we can see that it is the average of structures II and III. The triple and single bonds present in both these structures are represented as double bonds between all three nitrogen atoms in structure I. The total charges on the molecule can be adjusted by giving a single unit of negative charge on both the terminal nitrogen atoms. To put it in simpler terms, structure I is the resonance hybrid structure of the Azide ion. And we know that the stability of the hybrid structure is higher than all the other canonical structures and hence contributes the most to the stability of the given molecule.
Hence, Structures II and III make smaller contributions than I
Hence, Option B is the correct option
Note: Another way to explain the stability of the given resonance structures, is to evaluate the charge distribution on all the atoms in the resonance structure. If opposite charges are distributed on adjacent atoms, then the stability of those resonance structures is lower than the structures that have charges that are distributed by having neutral atoms between them.
