Question
Question: Azide ion \(\left( {N_3^ - } \right)\) exhibits on \(N - N\) bond order of \(2\) and may be represen...
Azide ion (N3−) exhibits on N−N bond order of 2 and may be represented by resonance structures I,II and III given below:
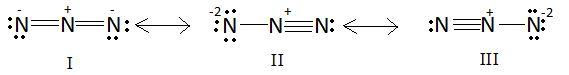
Select correct statements.
A: Structures I and II make greater contributions than III.
B: Structures II and III make smaller contributions than I.
C: Structures I and III make greater contributions than II.
D: All the three structures make equal contributions.
Solution
Resonance structures are also called canonical structures. Resonance is a way of describing bonding in chemical molecules. In resonance structures delocalization of charge takes place. This delocalization of charge makes the molecule more stable.
Complete step by step answer:
Azide is an anion. Anion is the species with negative charge. Chemical formula of azide is N3−. This ion is represented by some resonating structures. These structures are as follows:
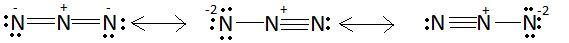
Out of these three structures, the first structure is the most stable. In the first structure there is a small charge on three nitrogen atoms but in the other two structures there is a −2 charge on a nitrogen atom which is quite large for a small atom like nitrogen. Also there is a triple bond present between two nitrogen atoms in the second and third structure due to which the molecule will tend to convert to nitrogen gas. Due to these reasons, the second and third structure is less stable as compared to the first structure. So, we can say that structures II and III make smaller contributions than I.
Therefore the correct answer is option B.
Additional information: In resonating structures, the position of all the atoms is the same in all the structures but the position of double bond, charge and lone pair may vary. A true resonating structure of a molecule is the structure which is the average of the resonating structure for that molecule.
Note:
Most stable resonance structure is called resonance hybrid. A molecule can have resonating structures when it has a lone pair or double bond on the atom next to a double bond. Resonating structures are used when there is more than one way to show the position of double bond or lone pair in a molecule.
