Question
Question: Atomicity of oxygen in ozone molecules is _________....
Atomicity of oxygen in ozone molecules is _________.
Solution
Atomicity of an element is defined as the total number of atoms present in one molecule of the element. Depending upon the number of atoms present in a molecule, it can be monatomic, diatomic, triatomic, or polyatomic.
Complete answer:
Ozone has the molecular formula O3. Ozone is another form of oxygen element.
We know that the atomicity is the total number of atoms present in one molecule. For example, oxygen is present in the atmosphere as O2. It has two oxygen atoms, therefore, its atomicity is 2.
Similarly, we can determine the atomicity of ozone. One molecule of ozone (O3) is composed of three atoms. Thus, the atomicity of oxygen in ozone is 3. In other words, we can say that ozone is a triatomic molecule.
Alternatively, we can also find out the number of oxygen atoms (i.e. atomicity) in ozone from the ratio of molecular mass of ozone to the atomic mass of oxygen., i.e.
Atomicity = atomicmassofOmolecularmassofO3
Atomic mass of oxygen = 16g
Molecular mass of O3 = 3×16 = 48g
Therefore, on dividing the two, we get atomicity = 16g48g=3.
Hence, atomicity of oxygen in ozone molecules is 3.
So, the correct answer is “Option D”.
Additional Information: Structure of ozone molecule is explained by resonance.
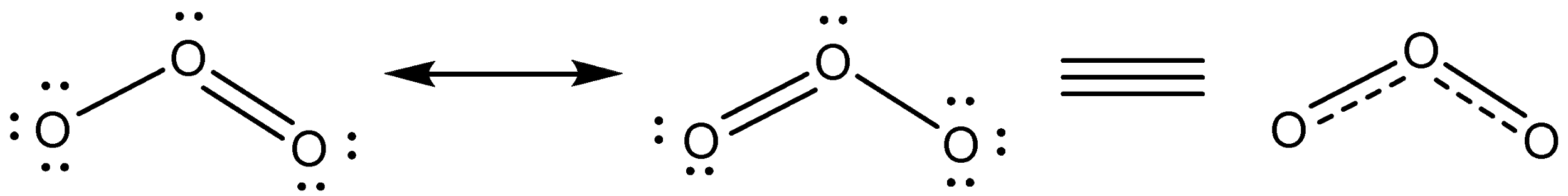
Ozone is found in the upper atmosphere. It is formed by the action of ultraviolet radiation on O2 . The high energy UV radiation splits O2 into oxygen atoms, which react with O2 to form O3.
