Question
Question: Atomic number of iron is 26. It exhibits \(F{e^{ + 2}}\), \(F{e^{ + 3}}\) oxidation state. Write the...
Atomic number of iron is 26. It exhibits Fe+2, Fe+3 oxidation state. Write the subshell electronic configuration.
Subshell electronic configuration
Fe
Fe+2
Fe+3
Solution
. To answer this question we should know about electronic configuration and rules to write the electronic configuration. Iron is a d-block element. It is very important to understand variable oxidation state.
Complete step by step answer:
Electronic configuration can be defined as the distribution of electrons in various shells, subshells and in the orbitals of an atom.
The rules to write electronic configuration:
- The number of electrons in a shell: According to Bohr-bury scheme, the maximum number of electrons a shell can hold is equal to 2n2, where n is the number of shells.
If n=1 , the maximum number of electrons this shell can hold is 2(1)2 that is two electrons.
-The number of electrons in sub- shell: The maximum number of electrons the subshell can hold is given by azimuthal quantum number which is equal to 2(2l+1), where l is 0,1,2,3 for s, p, d, f sub-shell respectively.
If l = 2,
= 2(2l+1)
= 2 [ 2(2) + 1]
= 2 (5)
= 10
d subshell can hold 10 electrons only
-Aufbau principle: According to Aufbau principle only the subshells are arranged in a sequence with respect to the energy level.
Aufbau principle states that “In the ground state of the atoms, the orbitals are filled in order of their increasing energies”. This principle is based on Hund’s rule of maximum multiplicity and Pauli Exclusion Principle.
According to Aufbau principle, the order in which the energies of the orbitals increases:
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d ,5p, 4f, 5d……………..
Let’s write the electronic configuration:
We know the atomic number of iron is 26. The s shell can accommodate 2 electrons, p shell can accommodate 6 electrons, d shell can accommodate 10 electrons and f can accommodate 14 electrons.
Now, follow the above rules and write electronic configuration.
Firstly write the order in which the energies of the orbitals increase as given by Aufbau principle. Then keep on filling the electrons a particular shell can accommodate.
The electronic configuration of Fe = 1s2,2s2,2p6,3s2,3p6,4s2,3d6
Fe = 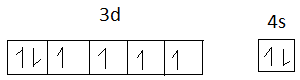
If two electrons are removed from the outermost shell then, it changes from Fe to Fe+2
The electronic configuration of Fe+2 = 1s2,2s2,2p6,3s2,3p6,4s0,3d6
Fe+2 = 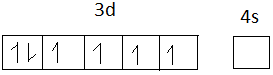
If three electrons are removed from the outermost shell, it changes from Fe to Fe+3
The electronic configuration of Fe+3 = 1s2,2s2,2p6,3s2,3p6,4s0,3d5
Fe+3 = 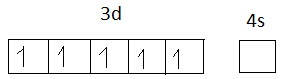
Note: The orbital diagram drawn above helps you understand the difference and remember. It’s important to understand the difference between shell and subshell. s,p,d,f are shells and 1s, 2s, 3s,….,2p, 3p, 4p…,3d, 4d, 5d…, 4f, 5f… are subshells.
