Question
Question: atmospheric pressure (in torr) should be: [Given : $K_b$ of water = 0.52 K-kg/mol] 4. A solution pre...
atmospheric pressure (in torr) should be: [Given : Kb of water = 0.52 K-kg/mol] 4. A solution prepared by dissolving 38.2 g of Na2B4O7⋅xH2O in 250 g water has boiling point 100.582°C. Find the value of x in the formula of salt. [Kb of the water is 0.52 K-kg mol−1, Atomic mass of B = 11, Na = 23]
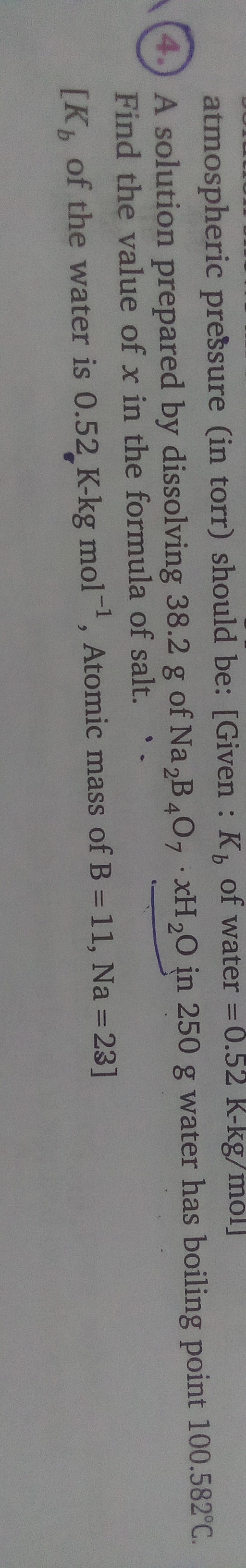
12
Solution
The problem asks us to find the value of 'x' in the hydrated salt Na2B4O7⋅xH2O using the elevation in boiling point data.
Here's a step-by-step solution:
-
Calculate the elevation in boiling point (ΔTb): The boiling point of the solution is 100.582∘C. The normal boiling point of pure water is 100∘C. ΔTb=Tb(solution)−Tb(pure water) ΔTb=100.582∘C−100∘C=0.582∘C (or 0.582 K, since a change in Celsius is equal to a change in Kelvin).
-
Determine the van't Hoff factor (i) for Na2B4O7: Sodium tetraborate (Na2B4O7) is an ionic compound that dissociates in water. Na2B4O7(aq)→2Na+(aq)+B4O72−(aq) For every one formula unit of Na2B4O7, 2 sodium ions and 1 tetraborate ion are produced, totaling 3 ions. Therefore, the van't Hoff factor, i=3.
-
Use the elevation in boiling point formula to find the molality (m) of the solution: The formula for elevation in boiling point is: ΔTb=i⋅Kb⋅m Given: ΔTb=0.582 K, Kb=0.52 K-kg/mol, i=3. 0.582=3⋅0.52⋅m 0.582=1.56⋅m m=1.560.582 m≈0.3730769 mol/kg
-
Calculate the moles of the solute (Na2B4O7⋅xH2O): Molality (m) is defined as moles of solute per kilogram of solvent. Mass of water (solvent) = 250 g = 0.250 kg. Moles of solute = m×Mass of solvent (in kg) Moles of solute = 0.3730769 mol/kg×0.250 kg Moles of solute ≈0.0932692 mol
-
Calculate the experimental molar mass of the hydrated salt: Mass of Na2B4O7⋅xH2O dissolved = 38.2 g. Molar mass = Moles of soluteMass of solute Molar mass ≈0.0932692 mol38.2 g Molar mass ≈409.56 g/mol
-
Calculate the theoretical molar mass of Na2B4O7⋅xH2O in terms of x: Atomic masses: Na = 23, B = 11, O = 16, H = 1. Molar mass of Na2B4O7: (2×23)+(4×11)+(7×16)=46+44+112=202 g/mol Molar mass of H2O: (2×1)+16=18 g/mol Molar mass of xH2O=18x g/mol Total theoretical molar mass of Na2B4O7⋅xH2O=202+18x g/mol
-
Equate the experimental and theoretical molar masses and solve for x: 202+18x=409.56 18x=409.56−202 18x=207.56 x=18207.56 x≈11.531
Since 'x' represents the number of water molecules of crystallization, it must be an integer. Rounding 11.531 to the nearest integer gives 12.
Explanation of the solution:
- Calculate ΔTb from the given boiling points.
- Determine the van't Hoff factor (i) for Na2B4O7, which dissociates into 3 ions.
- Use the colligative property formula ΔTb=i⋅Kb⋅m to find the molality (m).
- Calculate the moles of solute from molality and mass of solvent.
- Determine the experimental molar mass of the salt using its given mass and calculated moles.
- Express the theoretical molar mass of Na2B4O7⋅xH2O in terms of 'x' using atomic masses.
- Equate the experimental and theoretical molar masses and solve for 'x', then round to the nearest integer.
