Question
Question: At the temperature of liquefaction of air, the ratio of ortho and para hydrogen is: A)1:1 B)1:3 ...
At the temperature of liquefaction of air, the ratio of ortho and para hydrogen is:
A)1:1
B)1:3
C)3:1
D)3:2
Solution
To answer this question, you should recall the concept of the different spin of hydrogen nuclei that exist in nature. The molecule of dihydrogen consists of two atoms, in which the nuclei of both the atoms are spinning. Dihydrogen is of two types depending upon the direction of the spin of the two nuclei.
Complete Step by step solution:
We know that the Ortho hydrogen molecule is the one in which the spins of both the nuclei are in the same direction. Para hydrogen is the one in which the spins of both the nuclei are in the opposite direction Ordinary dihydrogen is an equilibrium mixture of ortho and para hydrogen. At the temperature of liquefaction of air, the ratio of ortho and para hydrogen is 1: 1. A diagrammatic representation of the nuclei can be shown as:
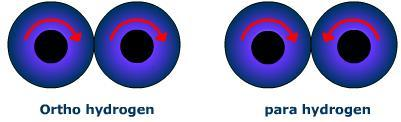
ortho hydrogen⇌para hydrogen
Therefore, we can conclude that the correct answer to this question is option A
Note: The amount of ortho and para hydrogen is a variable that depends on temperature as, At 0°K, hydrogen contains mainly para-hydrogen which is more stable. Room temperature changes this ratio of ortho to para hydrogen to 3: 1. This is the maximum possible ratio. This means that even at very high temperatures, the ratio of ortho to para-hydrogen can never be more than 3: 1. Thus, it has been possible to get pure para-hydrogen by cooling ordinary hydrogen gas to very low temperature but it is impossible to get a sample of hydrogen which contains more than 75% of ortho hydrogen.
