Question
Question: At the critical micelle concentration, the surfactant molecules: A.Decompose B.Disassociate ...
At the critical micelle concentration, the surfactant molecules:
A.Decompose
B.Disassociate
C.Associate
D.Become completely soluble.
Solution
Compare the structure of the surfactants and micelle, the changes in properties which happen due to the change in shape and correlate one by one if these changes can happen when the surfactant molecule undergoes the above mentioned options.
Complete answer:
Let us start by understanding what are surfactant molecules and micelles, and what is their relationship with the critical micelle concentration.
Surfactant molecules function exactly as their name suggests, these are surface acting molecules.They act at the interface of two phases, one phase in which they are dispersed in and the second phase is air. They will lower the surface tension between two phases by getting adsorbed on the interface of the two phases.
They have a hydrophobic part which is called the tail, this tail is made of a long hydrocarbon chain.
They have a hydrophilic part which is called the head , this head is made of a polar group and it is responsible for the solubility of the molecule.
Hence, these groups are called amphiphiles. Since they have both a polar and a non-Polar group.
When the concentration of the surfactants starts to increase in the medium, the molecules start to aggregate or associate. This new associate compound formed is known as a micelle.
Micelles are formed due to increased concentration of surfactant molecules, no more molecules can be accommodated at the surface and hence they undergo self-assembly to form an associated structure known as micelle. The concentration at which the change from surfactant to micelle takes place is called the critical micelle concentration.
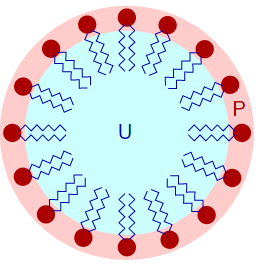
The group shown in red is the polar head and the blow zig-zag line represents the long hydrophobic hydrocarbon chain.
So the correct answer is option C.
Note:
Micellar colloids are in dynamic equilibrium with their monomers in the solution and as their concentration increases the shape of the micelle structure changes from spherical to cylindrical to hexagonal and then finally lamellar.
