Question
Question: At room temperature, sodium crystallises in a body centred cubic lattice with \(a=4.24{{A}^{\circ }}...
At room temperature, sodium crystallises in a body centred cubic lattice with a=4.24A∘ . Calculate the theoretical density of sodium. ( atomic mass of Na=23.0 )
A.1.002gcm−3
B.2.002gcm−3
C.3.003gcm−3
D.None
Solution
Body centred cubic lattice have lattice point at the centre of the cube and also to the eight corners of the cube. Body centred cubic lattice is a three dimensional lattice.
Formula used: Density=N∘×VZ×M
Where, Z Denotes as the no. of atom in a unit cell
M denotes as the molar mass
NA Denotes as the avogadro number
V denotes as the volume of the unit cell
Complete step by step answer:
Body centred cube has eight corners which is shared by eight cells and has one lattice at its centre.
Therefore, the no of atoms in a unit cell(N)=8×81+1=2
Here the atomic mass of sodium is 23
Volume of the unit cell =a3=(4.24×10−8)3cm3
ρ=NA×Vz×M
Where, ρ is the density
Z Is the number of atoms
NA Denotes as the Avogadro number
V denotes the volume of the unit cell
On substituting the values in the above given formula we get,
Hence, Density=(6.023×1023)(4.24×10−8)32×23
Density =1.002gcm−3
Therefore, the correct option is (A)
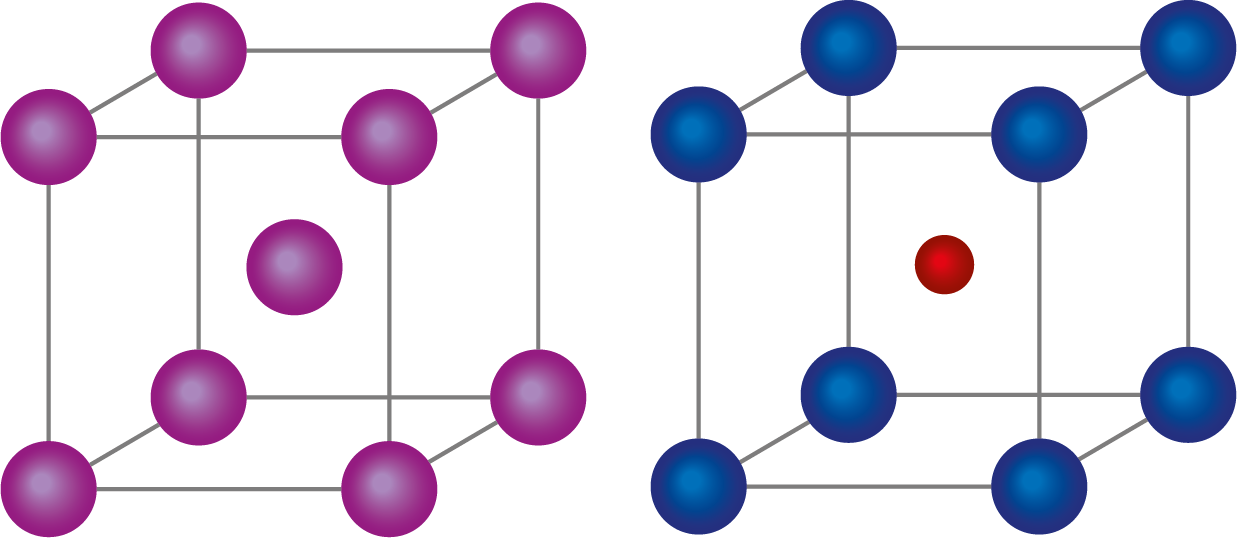
Body centred cubic lattice
Additional information
The packing efficiency of body centred cube is 68
The coordination no of an atom is 8
The void present in body centred cubic lattice is distorted octahedral
When the lattice gets inflated they touch each along the diagonals of the cube, so the radius of closed packed unit cell is 4r=3a
Note:
Unit cell is defined as the smallest group of atoms that can form an entire lattice by repetition of three dimensions.
Lattice is defined as the three dimensional in which a whole crystal is built.
Density of a unit cell is defined as mass per volume of the unit cell.
Packing efficiency is defined as the fraction of crystals occupied by the atoms and it should always be less than 100
