Question
Question: At \[400K\] energy of activation of a reaction is decreased by \[0.8kcal\] in the presence of a cata...
At 400K energy of activation of a reaction is decreased by 0.8kcal in the presence of a catalyst. Hence the rate will be
A.Increased by 2.71 times
B.Increased by 1.18 times
C.Decreased by 2.72 times
D.Increased by 6.26 times
Solution
The given reaction is an example of redox reaction and can therefore be separated into two half reactions where one chemical species is being oxidized and the other is being reduced. In order to balance the equations the number of electrons from each half reaction should be the same.
Complete answer:
Oxidation is the process of losing electrons and reduction is the process of accepting electrons. A redox reaction contains both procedures being carried out simultaneously by different chemical species.
Ion electron method of balancing redox reaction equations has the following set of rules:
1.Indicate oxidation numbers on top of each species to help identify oxidation and reduction halves of the reaction.
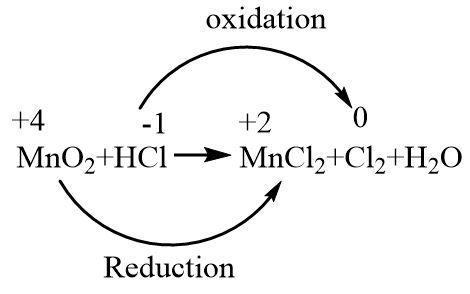
In the given reaction, MnO2 is being reduced to Mn2+ as the oxidation number decreases from four to two. Cl− is being oxidized to Cl2 as the oxidation number increases from negative one to zero.
2.Split the reaction into two half reactions where one is the oxidation reaction and the other is the reduction reaction and add electrons to balance the oxidation numbers.
The given reaction can be written as a combination of the following two reactions:
a.Oxidation
Cl−→Cl2+e−
b.Reduction
MnO2+2e−→Mn2+
3.Balance the half-reactions by making the number of atoms equal on both sides. The oxygen atoms are balanced by adding water molecules to the opposite side and the hydrogen atoms are balanced by adding an equal number of H+ on the opposite side.
MnO2+4H++2e−→Mn2++2H2O
The reduction half-reaction can be balanced by adding two molecules of water of the right side and adding four protons on the left side.
2Cl−→Cl2+2e−
The oxidation half-reaction can be balanced by balancing the number of chlorine atoms. If chloride ions are multiplied by two then the number of electrons also need to be doubled.
4.The electrons released in the oxidation process must be equivalent to the number of electrons accepted or gained in the reduction process hence multiplying the half reactions with a suitable number to get equal electrons in both half-reactions. Once the electrons are equal, add the two half-reactions to get the balanced final reaction.
MnO2+4H++2Cl−+2e−→Mn2++2H2O+Cl2+2e−
Since the electrons are already balanced, the two equations can be added. The derived equation can be rewritten by cancelling the electrons on both sides and adding 2Cl− on both sides to get chlorides of hydrogen and manganese ions present freely.
MnO2+4HCl→MnCl2+2H2O+Cl2
The equation written above is the final balanced redox equation.
Note:
Adding two extra chloride ions in the final step was not a mandatory step as the equation had already been balanced, it was only done to express the equation in the form of complete ionic compounds and not free ions. Adding 2Cl− on both the sides does not bring any change to the redox reaction.
