Question
Question: Assuming **2s-2p** mixing is not operative the paramagnetic species among the following is: A) \(\...
Assuming 2s-2p mixing is not operative the paramagnetic species among the following is:
A) Be2
B) N2
C) C2
D) B2
Solution
The magnetic property of a molecule can be explained based on the molecular orbital theory. The molecule which does not contain the unpaired electron is known as the paramagnetic. The molecule which has all-electron paid-up does not contribute towards the magnetic property. It is diamagnetic in nature. To solve such a problem write down the MOT diagram of molecules.
Complete step by step solution:
The MOT diagram is drawn in such a way that we do not consider the s and p orbital mixing. Therefore the order of orbitals is not changed.
Let’s first draw the MOT of all molecules given in the problem.
A) Be2 molecule: Beryllium atom has two electrons in the first shell and two-electrons in the second shell. The electronic configuration of the beryllium atom is as shown below,
Be = 1s2 2s2
The Be2 molecule contains a total of 8 electrons in it. These 8 electrons are arranged in the molecular orbitals.
The MOT is as shown below,
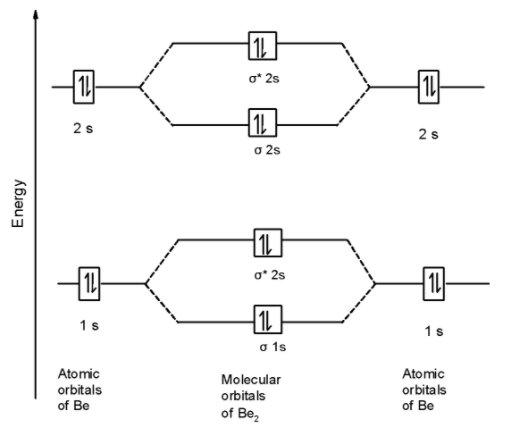
First of all, we can write the molecular orbital configuration of Be2 . The molecular orbital configuration of Be2 the molecule is as follows:
$$$$$$\text{ }\!\!\sigma\!\!\text{ 1}{{\text{s}}^{\text{2}}}\text{,}{{\text{ }\!\!\sigma\!\!\text{ }}^{\text{}}}\text{1}{{\text{s}}^{\text{2}}}\text{, }\!\!\sigma\!\!\text{ 2}{{\text{s}}^{\text{2}}}\text{, }{{\text{ }\!\!\sigma\!\!\text{ }}^{\text{}}}\text{2}{{\text{s}}^{\text{2}}}\text{ OR KK , }\!\!\sigma\!\!\text{ 2}{{\text{s}}^{\text{2}}}\text{, }{{\text{ }\!\!\sigma\!\!\text{ }}^{\text{*}}}\text{2}{{\text{s}}^{\text{2}}}\text{ }$$
Since, here all molecular orbitals contain the two electrons. These are all paired electrons. Thus Be2 the molecule is diamagnetic.
B) N2 molecule : The nitrogen atom has three electrons in the 2p shell and 4 electrons in the innermost shell. The electronic configuration of nitrogen atom is as shown below,
N = 1s2 2s2 2p1x= 2p1y=2p1z
The N2 molecule contains a total of 14 electrons in it. These 14 electrons are arranged in the molecular orbitals. The MOT is as shown below,
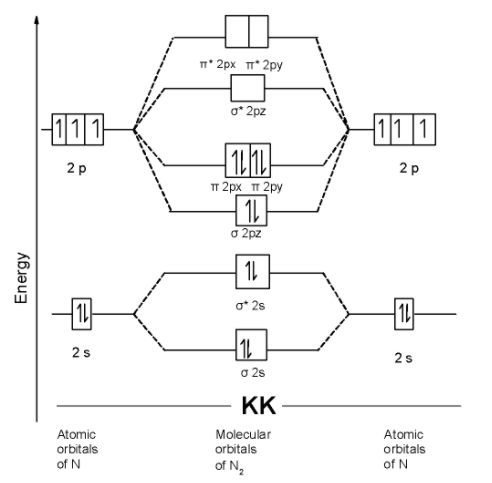
We can write the molecular orbital configuration of N2 . The molecular orbital configuration of Be2 the molecule is as follows:
!!σ!! 1s2, !!σ!! *1s2, !!σ!! 2s2, !!σ!! *2s2 , !!π!! 2py2 = !!π!! 2px2 , !!σ!! 2pz2
Since, here all molecular orbitals contain the two electrons. These are all paired electrons. Thus N2 the molecule is diamagnetic.
C) C2 Molecule: The carbon atom has two electrons in the 2p shell and 4 electrons in the innermost shell. The electronic configuration of nitrogen atom is as shown below, C = 1s2 2s2 2p1x= 2p1y=2p0z
The C2 molecule contains a total of 12 electrons in it. These 12 electrons are arranged in the molecular orbitals.
The MOT is as shown below,
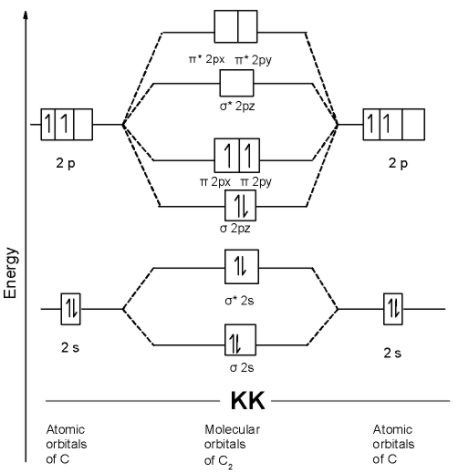
we can write the molecular orbital configuration of C2 . The molecular orbital configuration of C2 the molecule is as follows:
!!σ!! 1s2, !!σ!! *1s2, !!σ!! 2s2, !!σ!! *2s2 , !!σ!! 2pz2, !!π!! 2py1 = !!π!! 2px1
Since the pi molecular orbitals contain unpaired electrons. Thus C2 the molecule is paramagnetic.
D) B2 molecule: The boron atom has one electron in the 2p shell and 4 electrons in the innermost shell. The electronic configuration of nitrogen atom is as shown below,
B = 1s2 2s2 2p1x= 2p0y=2p0z
The B2 molecule contains a total of 10 electrons in it. These 10 electrons are arranged in the molecular orbitals.
The MOT is as shown below,
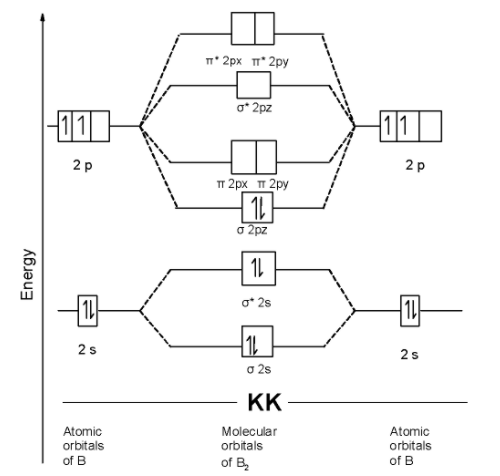
We can write the molecular orbital configuration of B2 . The molecular orbital configuration of B2 the molecule is as follows: !!σ!! 1s2, !!σ!! *1s2, !!σ!! 2s2, !!σ!! *2s2 , !!σ!! 2pz2
Since the molecular orbitals theory does not contain the unpaired electron. Thus B2 the molecule is diamagnetic.
Thus, from here we know that only C2 molecule is paramagnetic.
Hence, (C) is the correct option.
Note: Note that, in atoms like Li , Be , C , N the energy difference is very small between the 2s and 2p orbitals. These are close to each other and thus p and s orbitals combine and lead to change in the expected order of the orbital energies. Here, we are not considering the mixing of s and p orbitals thus the carbon molecule is paramagnetic otherwise if we consider the mixing then the carbon molecule is diamagnetic.
