Question
Question: Assign ON to atoms of only those elements which undergo ON change in the following redox reactions a...
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the equation.
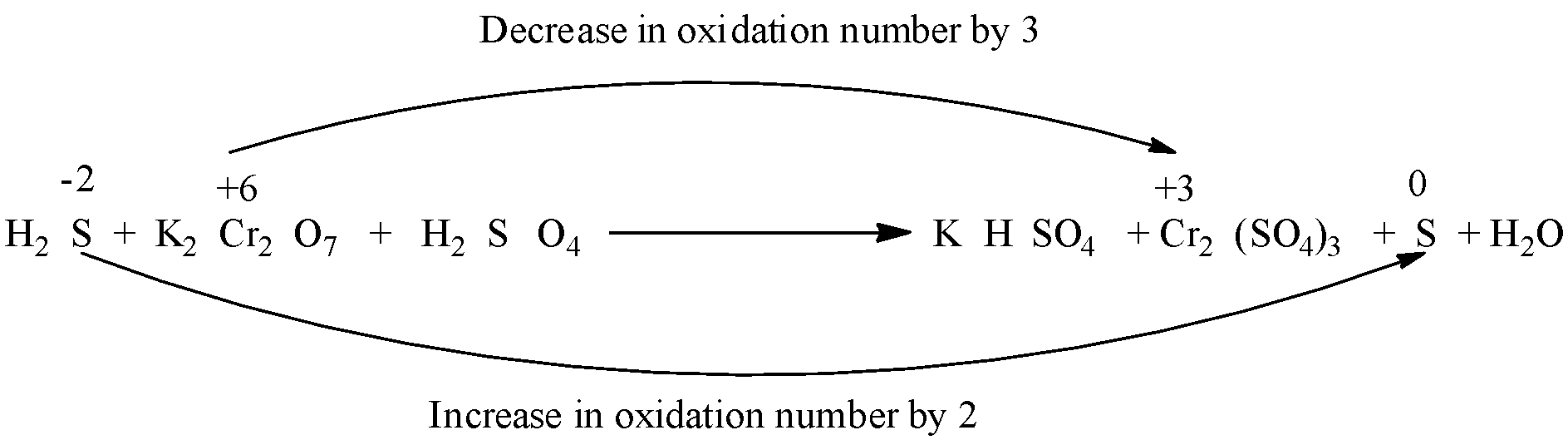
H2S+K2Cr2O7+H2SO4→KHSO4+Cr2(SO4)3+S+H2O
Solution
First, balance all the elements except hydrogen and oxygen atoms, so in this reaction balance sulfur, chromium, potassium before balancing hydrogen and oxygen. For finding the oxidation state of the elements, take the oxidation state of hydrogen and potassium as +1 and oxidation number oxygen as -2.
Complete answer:
The given reaction is:
H2S+K2Cr2O7+H2SO4→KHSO4+Cr2(SO4)3+S+H2O
The oxidation number of H in H2S is +1 and the oxidation number of S is -2.
The oxidation number of K in K2Cr2O7 is +1, the oxidation number of Cr is +6 and the oxidation number of O is -2.
The oxidation number of H in H2SO4 is +1 and the oxidation state of SO42− is -2.
The oxidation number of K and H in KHSO4 is +1, and the oxidation state of SO42− is -2.
The oxidation number of Cr in Cr2(SO4)3 is +3, and the oxidation state of SO42− is -2.
Since the S is in the elemental form its oxidation state will be 0.
The oxidation state of H in H2O is +1 and the oxidation state of O is -2.
So, in the reaction, the oxidation state of chromium decreases from +6 to +3, and the oxidation state of sulfur increases from -2 to 0. Therefore, H2S is the reducing agent and K2Cr2O7 is the oxidizing agent.
First, balance all the elements except hydrogen and oxygen atoms, so in this reaction balance sulfur, chromium, potassium before balancing hydrogen and oxygen.
So, the balanced equation will be:
3H2S+K2Cr2O7+5H2SO4→2KHSO4+Cr2(SO4)3+3S+7H2O
Note:
If the equation is in an acidic medium then to balance the hydrogen atoms, we can add hydrogen ions (H+) and if the equation is in basic medium then to balance hydrogen and oxygen we can add hydroxyl ions (OH−).
