Question
Question: Assertion: Trans-1,2-dimethylcyclopropane is a chiral molecule. Reason: All chiral molecules have ...
Assertion: Trans-1,2-dimethylcyclopropane is a chiral molecule.
Reason: All chiral molecules have chiral carbon atoms.
(A) Both assertion and reason are correct and the reason is the correct explanation for assertion.
(B) Both assertion and reason are correct but the reason is not the correct explanation for assertion.
(C) Assertion is correct but reason is incorrect.
(D) Both assertion and reason are incorrect.
Solution
A atom (which is usually tetrahedral) of a molecule containing four different bonded groups, including lone pairs, is known as a chiral center or a chiral atom. They have a non-superimposable mirror image.
Complete answer:
The structure of a molecule can help in identifying the chiral centers. While looking at a structure of a molecule, look for an atom that is bonded through a single bond to four distinct ligands.
Now, the structure of trans-1,2-dimethylcyclopropane is
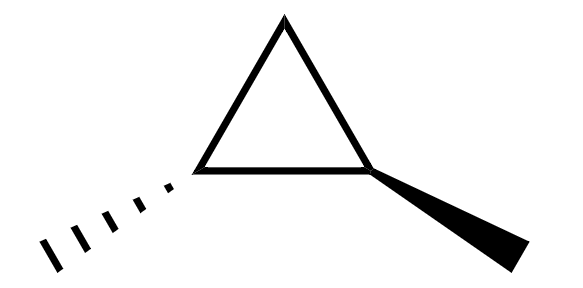
Here, we can see that both the carbon atoms which are attached to methyl groups have four different bonded groups, when looked at from the above,
1. hydrogen atom
2. methyl group
3. ring going clockwise
4. ring going anti-clockwise
Hence it is a chiral molecule.
Now, it is not necessary for a chiral molecule to have a chiral carbon or a center of chirality. Some of these chiral molecules are dimethylallene, dichloro spiro heptane, dibromobiphenyl, or trans-cyclooctene.
So, since the assertion is right but the reason is incorrect.
Hence the answer is option (C).
Additional Information:
Even though the terms chiral center and stereogenic center or stereocenter are used synonymously, not all stereocenters are considered to be chiral centers. But since stereocenters are bonded to three or more distinct groups, all chiral centers are stereocenters.
Note:
It should be noted that any atoms which have two or more identical groups attached to them cannot be a chiral center. For example, (−CH2 or -CH3).
A double-bonded or triple bonded carbon atom cannot be a chiral center as it can not form four distinct ligands.
