Question
Question: Assertion The radial probability distribution curves of \(1s\) , \(2p\) , \(3d\) - orbitals are id...
Assertion
The radial probability distribution curves of 1s , 2p , 3d - orbitals are identical in shape.
Reason
A.The number of nodal planes present in these orbits is different.
B.Both assertion and reason are correct and reason is the correct explanation for assertion
C.Both assertion and reason are correct but reason is not correct explanation for assertion
D.Assertion is correct but reason is incorrect
E.Assertion is incorrect but reason is correct
Solution
We need to remember that the electron density at a radial distance from the atomic nucleus is given by the radial distribution curve. 4πr2ψ2 (radial probability density function) value becomes 0 at a nodal point, it is also called a radial node. Radial nodes are equal to n−l, where, l is the azimuthal quantum number and n is the principal quantum number.
Complete step by step answer:
We must have to know that the nodal planes are locals around the nuclear cores where the probability of discovering electrons is zero. The direction of these planes are found by settling the Schrodinger wave condition particles or atoms to discover the state of nuclear and subatomic orbitals.
Let us find the assertion and reason are correct or not one by one to find the correct option.
Assertion:
The given orbitals are 1s , 2p , 3d . The principal quantum number and azimuthal quantum number are related as l=n−1 . Using the formula, radial nodes are equal to n−l , where, l is the azimuthal quantum number and n is the principal quantum number.
The principal quantum number (n) value for 1s , 2p & 3d orbitals are 1 , 2 , & 3 .
The azimuthal quantum number (l) value for 1s , 2p & 3d are 0 , 1 & 2 .
For 1s orbital the radial nodes are equal to 1 - 0$$$ = 1$.
For $2p$ orbital radial nodes are equal to n - l$$ =2−1=1.
Similarly for 3d orbital, 3−2=1 .
Radial nodes for 1s , 2p & 3d orbitals equal to 1, this shows the radial probability distribution curves of 1s , 2p & 3d orbitals are identical in shape.
Let us see the reason:
Reason says the number of nodal planes present in these orbits is different. First find out the nodal planes.
Nodal planes = l.
The l values for s,p&d are 0,1&2.
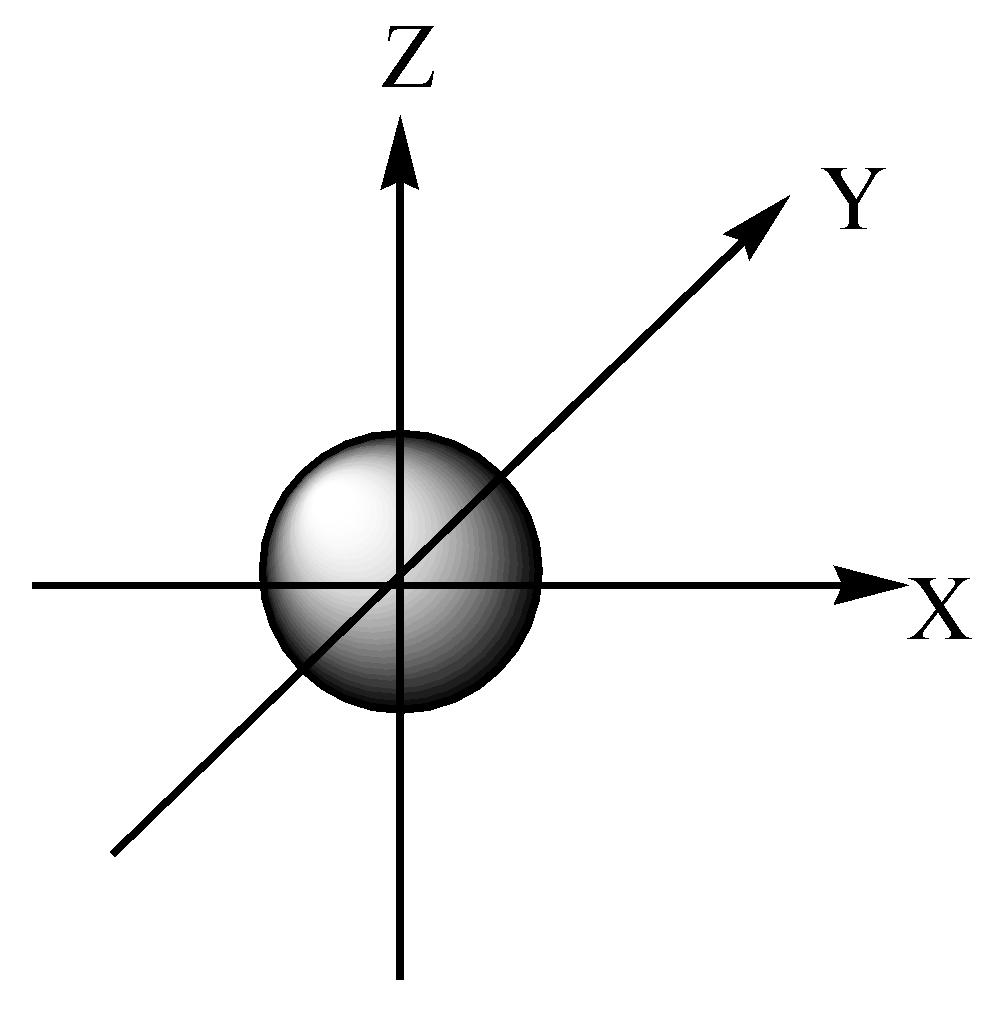
s orbital is spherical in shape and there is no nodal plane.
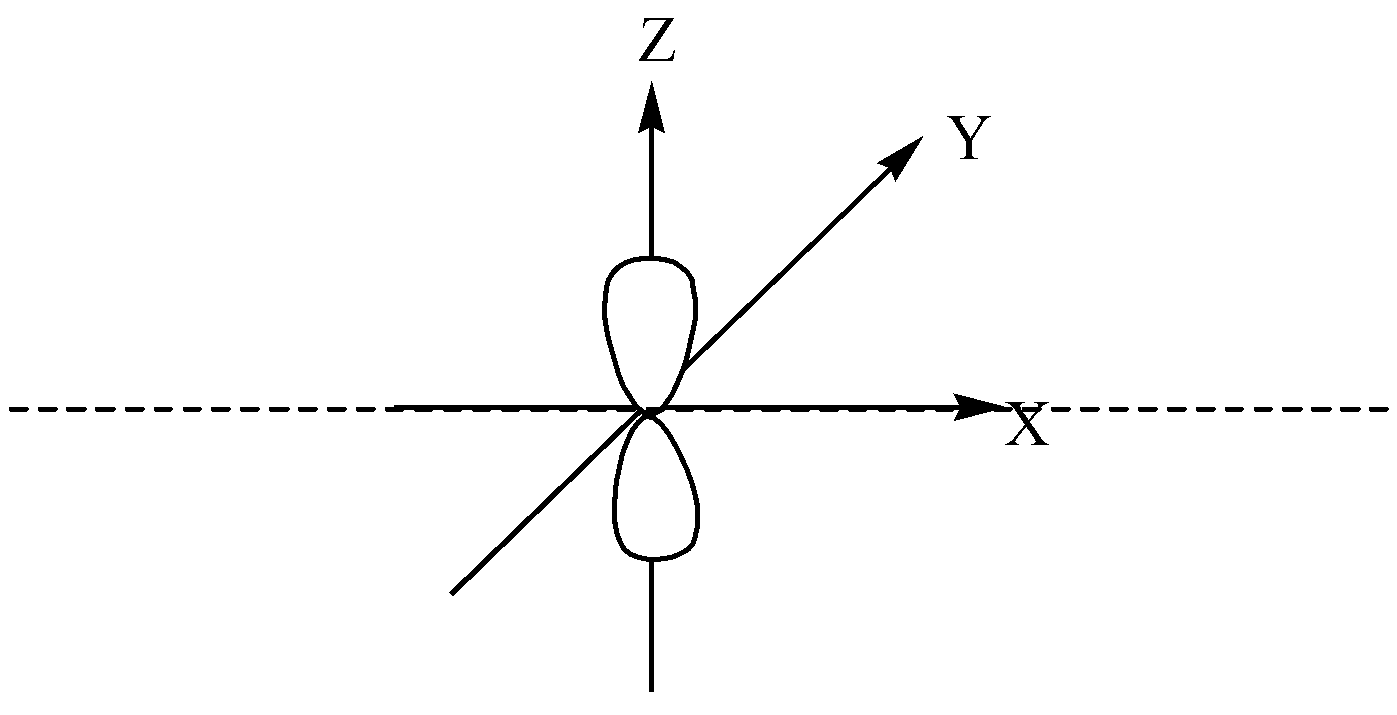
p orbital is dumbbell-shaped and has two lobes, there is a nodal plane.
d orbital is clover in shape and it has two nodal planes.
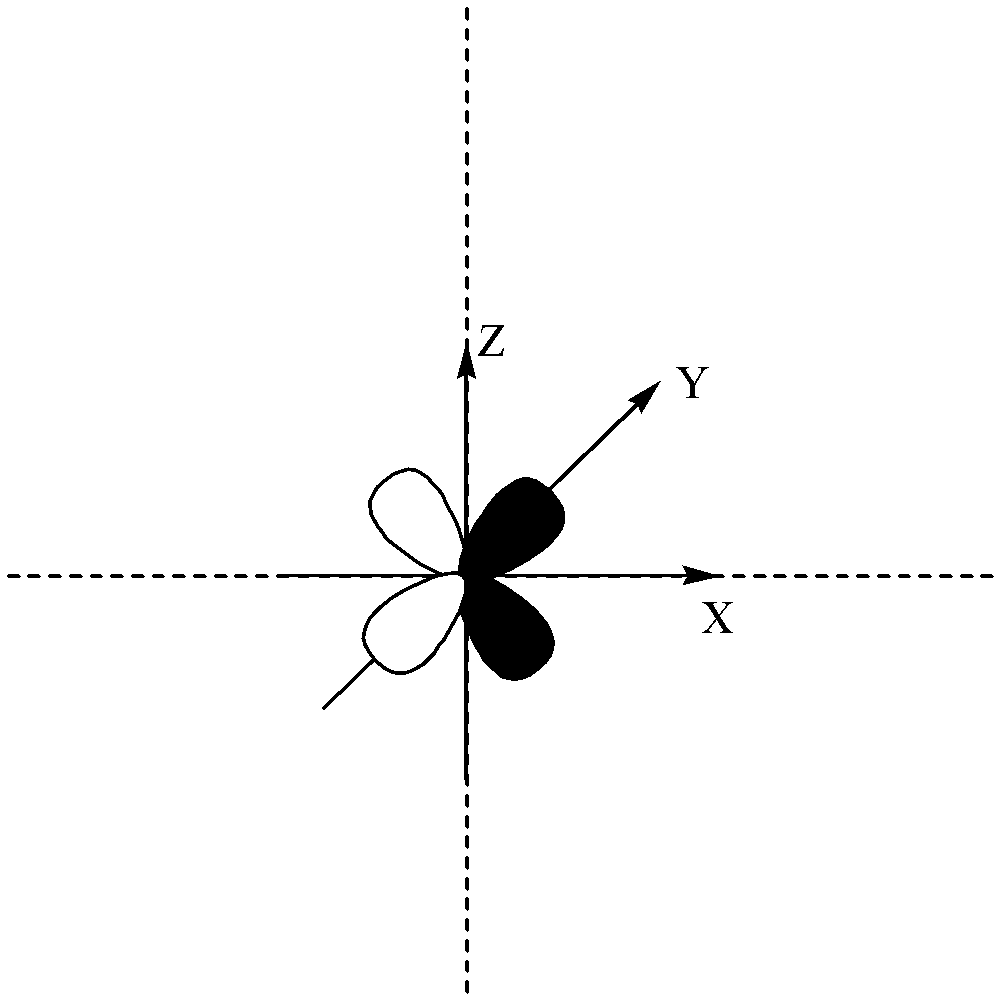
The number of nodal planes present in the above orbital is different.
Option B. both assertion and reason are correct but reason is not correct explanation for assertion is the correct answer.
Note:
We need to remember that the nodal surface (also called a radial node), which is a hollow sphere region in which electrons can’t be. A nodal plane (also called an angular node), which is either a plane, where electrons can’t be, or a conic surface ( dz2 orbital). For determining the electron configuration of an atom, quantum numbers are used. And also used for the probable location of the atom’s electron.
