Question
Question: Assertion: The order of stability of carbocation is \({{3}^{0}}>{{2}^{0}}>{{1}^{0}}\) Reason: Carb...
Assertion: The order of stability of carbocation is 30>20>10
Reason: Carbon atom in carbocation is in sp3 state of hybridization.
Solution
Draw the structure for the three types of carbocation. The carbocation which has least partial positive charge is more stable as the charge is distributed among neighbouring carbons. More the charge density on the carbon atom, the more unstable it is.
Complete step by step answer:
We will draw the structure of 30,20and10 to understand the factors affecting the stability of the carbocation.
Structure of 10 carbocation:
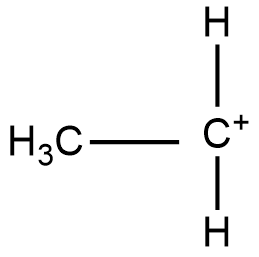
Structure of 20 carbocation:
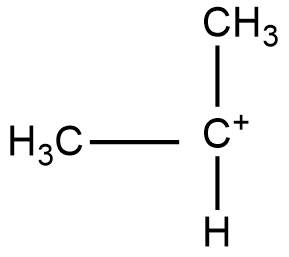
Structure of 30 carbocation:
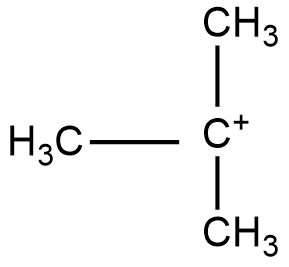
In the above compounds there is no possibility of resonance. So, we will check for greater inductive effect in the three carbocations.
-Inductive effect is an effect regarding the transmission of unequal sharing of the bonding electron through a chain of atoms in a molecule, leading to a permanent dipole in a bond.
The CH3−branch shows inductive effect by pushing electron density towards the carbon having positive charge thereby reducing charge density on the carbocation.
So, the carbocation with the greatest number of CH3− will be most stable. Hence, the order of stability of carbocation is 30>20>10.
Therefore, the assertion is correct.
The carbon atom in carbocation of is in sp3 state of hybridization for 30,20and10 carbocation.
Therefore, the reason is also correct however the reason for stability of carbocation is not the hybridization.
So, the correct answer is “Option B”.
Note: It is important to draw the structure of carbocation to understand the electronic effects which can be applied to stabilise the compound. Do not get confused between the reason being correct and the reason justifying the assertion as they are two different things.
