Question
Question: Assertion: The \(O - O\) bond length in \({H_2}{O_2}\) is shorter than that of \({O_2}{F_2}\). Rea...
Assertion: The O−O bond length in H2O2 is shorter than that of O2F2.
Reason: H2O2 has an open book-type structure.
Read the above assertion and reason and choose the correct option regarding it.
A.) Assertion is true, Reason is true and Reason is correct explanation for Assertion.
B.) Assertion is true, Reason is true and Reason is NOT the correct explanation for Assertion.
C.) Assertion is true, Reason is false.
D.) Assertion is false, Reason is true.
Solution
As both the compounds H2O2 and O2F2 have similar structure but the hydrogen peroxide that is H2O2 is a covalent compound and in O2F2 fluorine atoms are highly electronegative.
Complete step by step answer:
In this question, H2O2 is known as hydrogen peroxide and O2F2 is known as dioxygen difluoride. The hydrogen peroxide contains oxygen-oxygen single bond. Hydrogen peroxide has an open book structure, it is called so because it looks like an open book . It is a non-planar structure. There are two planes in the structure of hydrogen peroxide in which each plane has one O−H bond pair. The open book structure of hydrogen peroxide can be represented as:
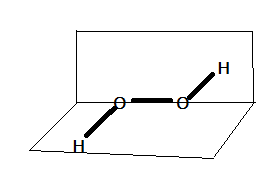
The structure of dioxygen difluoride (O2F2) is also similar to that of hydrogen peroxide that is dioxygen difluoride also have open book like structure but the bond length of oxygen-oxygen bond (O−O) is shorter in case of dioxygen difluoride. As we know that Florine is a strongly electronegative element and being a strongest electronegative element, it has tendency to attract electron density towards itself. Therefore, in O2F2 Florine attracts the electron density towards itself which results in smaller O−O bond.
As we know that O−O bond length in H2O2 is longer as that of in O2F2. Therefore, the Assertion is incorrect. Also, the Hydrogen peroxide has an open book structure. Therefore, Reason is true.
Hence, option D is the correct answer.
Note: Remember that in hydrogen peroxide (H2O2) oxygen also attracts the electron density towards itself but it does not attract to the extent of fluorine in O2F2. This is because Florine is more electronegative than oxygen.
