Question
Question: ASSERTION The isothermal curves intersect each other at a certain point. REASON The isotherma...
ASSERTION
The isothermal curves intersect each other at a certain point.
REASON
The isothermal changes take place rapidly, so the thermal curves have very little slope.
a.) Both Assertion and Reason are correct and Reason is the correct explanation for Assertion.
b.) Both Assertion and Reason are correct and Reason is not the correct explanation for Assertion.
c.) Assertion is correct but Reason is incorrect
d.) Both Assertion and Reason are incorrect
Solution
In order to solve the given problem and to check for the truth of the given assertion and the reason first of all we will try to understand the meaning of and isothermal process along with an example we will also see a general curve for isothermal process further we will first check for the truth of the assertion and further if the assertion is found to be correct then we will proceed forward to find the truth of the reason and we will select the correct option from the given options.
Complete step by step answer:
Let us first understand about the isothermal process.
The term isothermal corresponds to constant temperature.
A change in a system in which the temperature remains constant is an isothermal process. Usually, this happens while a system is in contact with an exterior thermal reservoir (heat bath), and the system adjustment can occur slowly enough to allow the system to begin to respond by heat exchange to the temperature of the reservoir.
In any form of device that has any means of temperature management, including highly engineered devices, and even living cells, isothermal processes may occur. Any portions of the cycles of such heat engines are isothermal. Isothermal cycles are also step variations, such as melting or evaporation.
Now let us see the isothermal process with the help of graphs by interpreting the ideal gas equation. The following graph represents an isothermal process in a P-V graph.
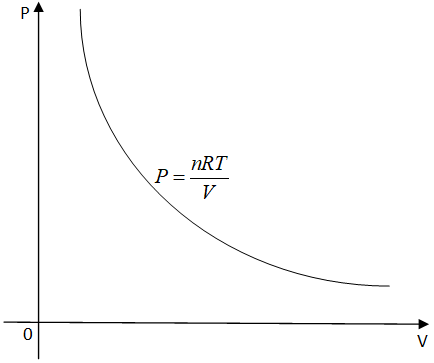
In order to see the different graphs, a perfect gas is compressed or allowed to expand very slowly to do isothermal production.
As we know that the isothermal curves will start at the same point and have very low slopes so the graphs will never intersect each other.
As the graphs will never intersect each other so the assertion is wrong. So the reason for the wrong assertion is also incorrect.
Hence, for the given assertion and reason both Assertion and Reason are incorrect.
So, the correct answer is “Option D”.
Note: In order to solve the given problem students must take care that as the given assertion is wrong so there is no need to check for the reason. Students must remember the characteristics of ideal gas and also the behavior shown by ideal gas under different physical conditions. In order to solve the “MCQ” problem the students must keep in mind the options as well.
