Question
Question: Assertion The hardness of water is determined by titrating it with disodium salt of EDTA Reason ...
Assertion
The hardness of water is determined by titrating it with disodium salt of EDTA
Reason
The indicator used in the titration is Eriochrome black-T at pH =10
Read the above assertion and reason and choose the correct option regarding it.
A.Both assertion and reason are correct and the reason is the correct explanation for assertion
B.Both assertion and reason are correct but the reason is not the correct explanation for assertion
C.The assertion is correct but the reason is incorrect
D.Both assertion and reason are incorrect
Solution
Hardness of water can be permanent and temporary. Permanent hardness is removed by titrating it against EDTA. The hardness of water can be estimated by many methods such as EDTA (ethylenediaminetetraacetic acid) titration, atomic absorption, gravimetric analysis, etc.
Complete answer:
Hardness of water is a measure of its capacity to precipitate soap and is caused mainly by the presence of calcium and magnesium. The content of calcium and magnesium is very high in hard water.
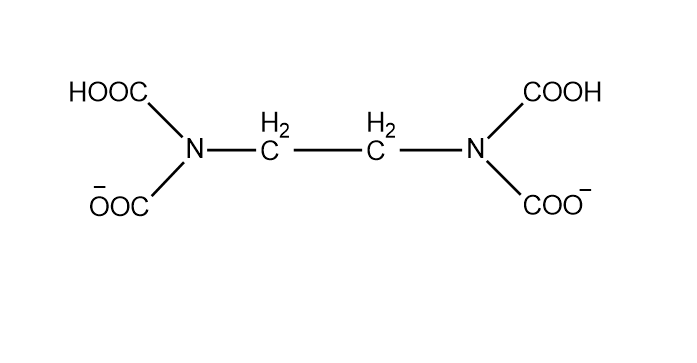
The most common method for determination of permanent hardness of water is by titrating it with a standard solution of disodium salt of EDTA. The indicator used in this titration is the Eriochrome Black T (EBT) indicator. During the titration EBT indicator is added to hard water. On addition EBT forms an unstable wine-red colored complex with the metal ion (Ca2+and Mg2+) at a pH of about9−10.
M2+(Ca2+or Mg2+)+EBTpH=9−10less stable (wine - red)[M−EBT]complex
After this when EDTA is added in the solution, the metal ion leaves the indicator and forms a stable metal-EDTA complex. When all the metal ions are taken by EDTA from the indicator-metal ion complex, the color of the solution changes from wine-red to steel blue. This indicates the end point of the titration. The reaction of the titration is:
(wine red)[M−EBT]+EDTApH=9−10more stable (colourless)[M−EDTA]complex+(steel blue)EBT
Therefore both assertion and reason are correct and reason is the correct explanation for assertion.
So the correct option is A.
Note:
The hardness of water can be temporary as well as permanent. Temporary hardness is due to bicarbonate ions, which can be easily removed by heating water. Permanent hardness is due to chlorides and sulphates of Ca2+,Mg2+,Fe3+and SO4−and it cannot be easily removed by boiling. So for removing them methods like titration against disodium salt of EDTA is used. EDTA is a chelating ligand.