Question
Question: Assertion: The ground state electronic configuration of nitrogen is as shown in the diagram. ![]...
Assertion:
The ground state electronic configuration of nitrogen is as shown in the diagram.
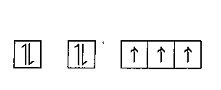
Reason:
Electrons are filled in orbitals as per Aufbau principle. Hund’s rule of maximum spin multiplicity and Pauli’s principle.
A. Both assertion and reason are correct and Reason is the correct explanation for Assertion.
B. Both assertion and reason are correct but Reason is not the correct explanation for Assertion.
C. Assertion is correct but Reason is incorrect.
D. Assertion is incorrect but Reason is correct.
Solution
Shells are energy levels around the nucleus of an atom. These are sub-divided into sub-energy levels called sub-shells. Sub-shells are named as s, p, d, f, etc. Every sub-shell or orbit has a fixed number of orbitals. Every orbital has fixed capacity to place electrons.
Complete step by step answer:
Aufbau principle states that when electrons organize themselves in various energy shells or orbits, they occupy the lower energy levels first and gradually make their way to higher energy levels. Some elements do not follow the general principle of Aufbau principle because for such elements, stability is determined by the presence of either a half-filled orbital or a fully filled orbital.
Pauli’s principle or exclusion principle states that each orbital can hold a maximum of two electrons. The two electrons will have opposite spins. The two spins are in clockwise and anti-clockwise direction or simply up and down. It is responsible for the chemical properties of elements.
Hund’s rule tells that when you get orbitals having the same energy, you fill them half way first, then start pairing.
Therefore Assertion and Reason are correct and Reason is the correct explanation for Assertion.
Additional information- If Pauli’s exclusion principle were not valid, an atom could radiate energy until every electron in the atom is in the lowest possible energy state. Therefore chemical behavior of the elements would be grossly modified. These principles are responsible for describing the electronic structure of an atom.
Note:
Electrons are assigned to orbitals using these three principles. The actual sequence of energy levels is given below:
1s,2s,2p,3s,3p,4s,3d,4p,5s,4d,5p,6s,4f,5d,6p, and so on.
