Question
Question: Assertion: The electronic configuration of nitrogen atoms is represented by the given figure. Reas...
Assertion: The electronic configuration of nitrogen atoms is represented by the given figure.
Reason: The electronic configuration of the ground state of an atom is the one which has greatest multiplicity.
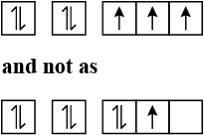
A. Both Assertion and Reason are correct and Reason is the correct explanation for Assertion.
B. Both Assertion and Reason are correct but Reason is not the correct explanation for Assertion.
C. Assertion is correct but Reason is incorrect.
D. Both Assertion and Reason are incorrect.
Solution
Electronic configuration tells us about the distribution of electrons of an atom or molecule in atomic or molecular orbitals. Electronic configuration was first discovered through the Bohr model of the atom which generally describes shells and subshells.
Complete answer:
Nitrogen atom have atomic number 7 and the distribution of electron is based on the three principles called Aufbau principle, Pauli Exclusion Principle and Hund’s rule which can be described as follows:
1. Aufbau principle: It states that electrons fill lowest atomic orbitals first after their completion and it moves towards higher level atomic orbitals. The subshell ordering according to this rule is given by: 1s,2s,2p,3s,3p,4s,3d,4p,5s,4d,5p,6s,4f,5d,6p,7s,5f,6d,7p,8s,5g...
2. Pauli Exclusion Principle: It states that no electrons in an atom are allowed to have the same set of quantum numbers.
3. Hund’s rule: This rule states that before the double occupation of any orbital it firstly singly occupied.
Thus according to Hund’s rule we can say that option A is the correct answer.
Note:
The electron distribution in electronic configuration firstly states the maximum number of electrons in their shells like s subshell acquire at most 2 electrons, p subshell have 6, d have 10 electrons, f subshell have 14 electrons and g subshell acquire 18 electrons in its orbit.
