Question
Question: Assertion: The \(B-F\) bond lengths in \(B{{F}_{3}}\) and \(B{{F}_{4}}^{-}\) are different Reason:...
Assertion: The B−F bond lengths in BF3 and BF4− are different
Reason: In BF3, the B−F bond acquires some double bond character.
A. Assertion and reason are true, reason is correct explanation for assertion
B. Assertion and reason are true, reason is correct explanation for assertion.
C. Assertion is true, reason is false.
D. Assertion is false, reason is true
Solution
BF3 has resonance structures, so it has a partial double bond character, whereas there is no resonance structure in BF4−ion, so it acts like single bond. Strength of a double bond is more than a single bond.
Complete answer:
In order to answer our question, we need to learn about the structures of BF3 and BF4−. BF3 is also known as bromine trifluoride and it has a total of 24 valence electrons. So, according to the VSEPR theory, the structure should be a triangle, with the boron atom, in the centre. The compound is sp2 hybridised. It happens as the s and the p orbitals of the Boron atom experience a combination, so that three sp2 hybrid orbitals are formed, all of which possess equal hybrid energy. The structure of BF3 is:
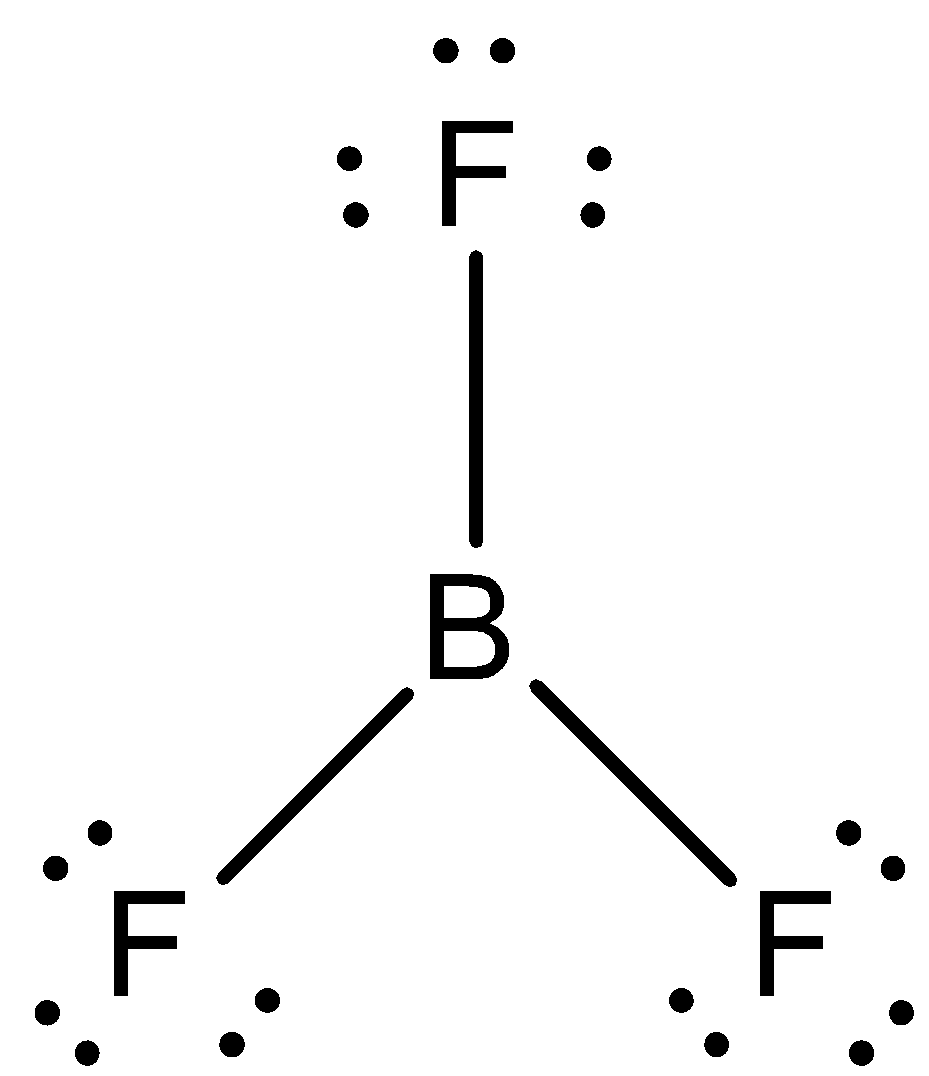
One electron of the boron in the ground state, remains unpaired. One 2s and two of the 2p orbitals hybridise. Now, let us know about the compound BF4−. It is also known as the boron tetrafluoride ion and has the structure of a tetrahedron. Each of the 3 valence electrons of boron forms a covalent bond with all of the four fluorine atoms. One more electron is contributed by the overall negative charge of the molecule. The structure of BF4− is:
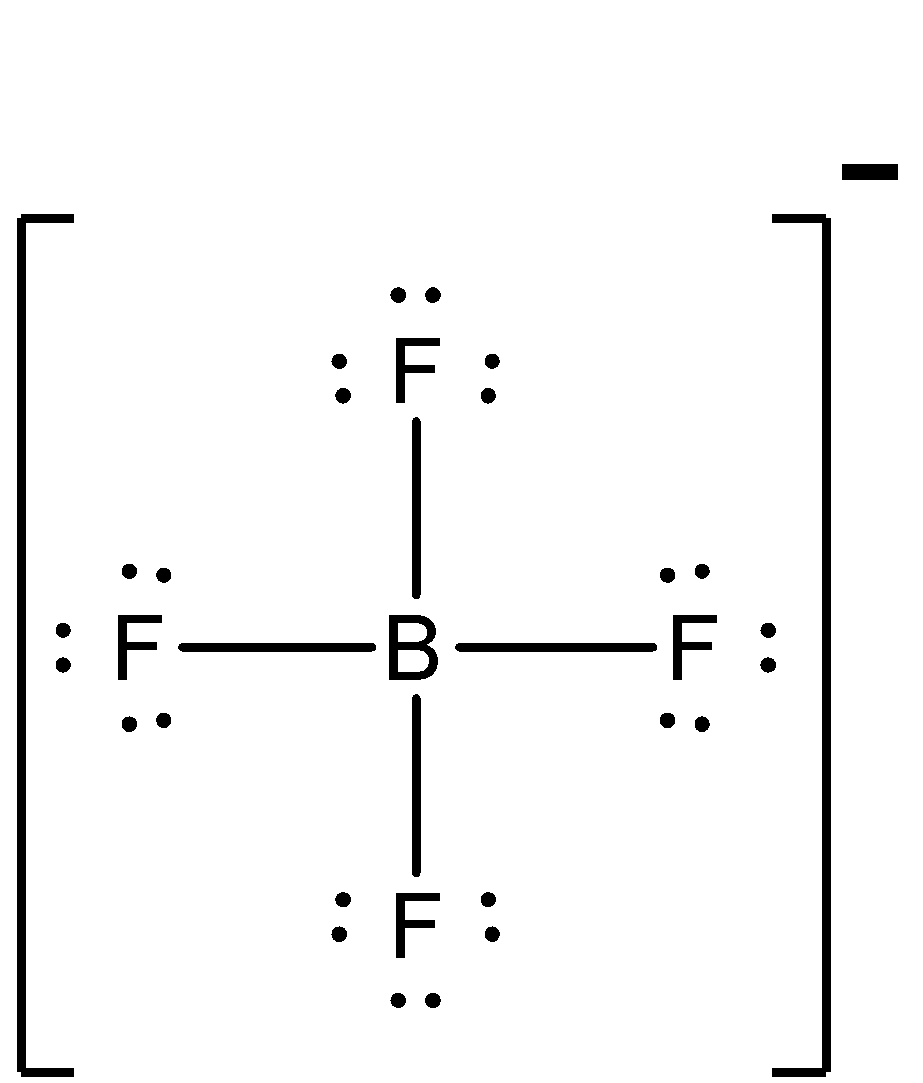
Now, BF4− has some single bond character, whereas BF3 has partial double bond character. So, the bond length of BF4− will be more, as bond strength is less.
So, the assertion and reason are true, reason is the correct explanation for assertion, which gives the correct answer as option A.
Note:
Boron trifluoride is a colourless and toxic substance which is generally present in the gaseous state. Fluorine is electronegative, but due to the arrangement of the fluorine atoms, the dipoles cancel out and net dipole becomes 0.
