Question
Question: Assertion:\[{\text{N(C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{N}}{{\text{H}...
Assertion:N(CH2CH2NH2)3 and EDTA are examples of polydentate ligands.
Reason :The ligand which can ligate through two different atoms is called a polydentate ligand.
A) Both Assertion and Reason are correct and Reason is the correct explanation for Assertion
B) Both Assertion and Reason are correct and Reason is not the correct explanation for Assertion
C) Assertion is correct and Reason is incorrect
D) Both Assertion and Reason are incorrect
Solution
Any atom , ion or molecule which is capable of donating a pair of electrons to the central atom is called a ligand. The ligand containing only one donor atom is known as a monodentate ligand. The ligand containing two donor atoms is known as a bidentate ligand. The ligand containing more than two donor atoms is known as a polydentate ligand.
Complete answer:
Assertion given to us is
N(CH2CH2NH2)3 and EDTA are examples of polydentate ligands.
To verify the given assertion we have to determine the number of donor atoms in each ligand.
The structure of the ligand N(CH2CH2NH2)3 is as follows:
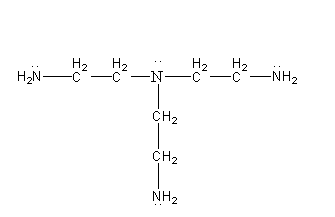
The ligand N(CH2CH2NH2)3 has 4 nitrogen atoms with lone pair on it so we can say that there are 4 donating atoms present inN(CH2CH2NH2)3 so it is a polydentate ligand.
Similarly, now we will determine the number of donating atoms in EDTA by drawing its structure.
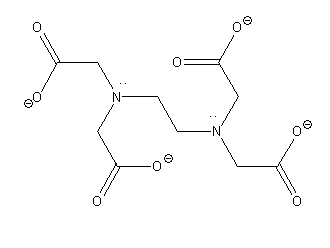
EDTA has 4 negatively charged oxygen atoms and two nitrogen atoms that is a total of 6 donor atoms. so it is a polydentate ligand.
Thus, we can say that the assertion N(CH2CH2NH2)3 and EDTA are an example of polydentate ligands is correct.
The reason given to us is the ligand which can ligate through two different atoms is called a polydentate ligand.
We know that the ligand which can ligate through two different atoms is called a bidentate ligand while the ligand which can ligate through more than two different atoms is known as a polydentate ligand.
Hence, we can say that the reason is incorrect.
Thus, the correct option is (C) Assertion is correct and Reason is incorrect.
Note: In a ligand, the atom which actually donates the electron pairs is called the donor atoms. The number of donor atoms determines the types of ligands that are monodentate, bidentate or polydentate ligand.
