Question
Question: Assertion \[S{O_3}\] has a planar structure. Reason Sulphur atom in \[S{O_3}\] is \[S{p^2}\...
Assertion
SO3 has a planar structure.
Reason
Sulphur atom in SO3 is Sp2 hybridized and O−S−O bond angle in 120∘
A.If both assertion and reason are CORRECT and reason is the CORRECT explanation of the assertion.
B.If both assertion and reason are CORRECT, but reason is NOT THE CORRECT explanation of the assertion.
C.If the assertion is CORRECT, but the reason is INCORRECT.
D.If assertion is INCORRECT, but reason is CORRECT.
Solution
We must have to know that the chemical compound with the formula SO3 is sulphur trioxide, with a relatively small liquid range. This species is a major pollutant in its gaseous form, being the primary precursor to acid rain. As a precursor to sulfuric acid, it is prepared on an industrial scale and is also known as sulfuric anhydride.
As we know that the shape of a molecule depends on the atoms which make up it and the electrons belonging to the central atom. The molecule is planar if the atoms organize themselves around the central molecule such that they reside on a single two-dimensional plane.
Complete step by step answer:
We know that the trigonal planar is a molecular geometry model of chemistry with one atom at the centre and three atoms at the corners of an equilateral triangle, referred to as peripheral atoms, all in one plane. All three ligands are equal in an ideal trigonal planar species and all bond angles are 120∘.
Sulfur trioxide is known to be a sulfuric acid anhydride. The trigonal planar structure of SO3 has a bond angle of 120∘. The SO3 molecule representation is shown below. The triangular planar molecule SO3 in the gas phase requires sp2 hybridization of the S atom. There are three sigma bonds formed by overlap of sp2−p and three pi bonds, one by overlap of p(π)-p(π) and two by overlap of p(π)-d(π). The bond angles of O−S−O are 120∘.
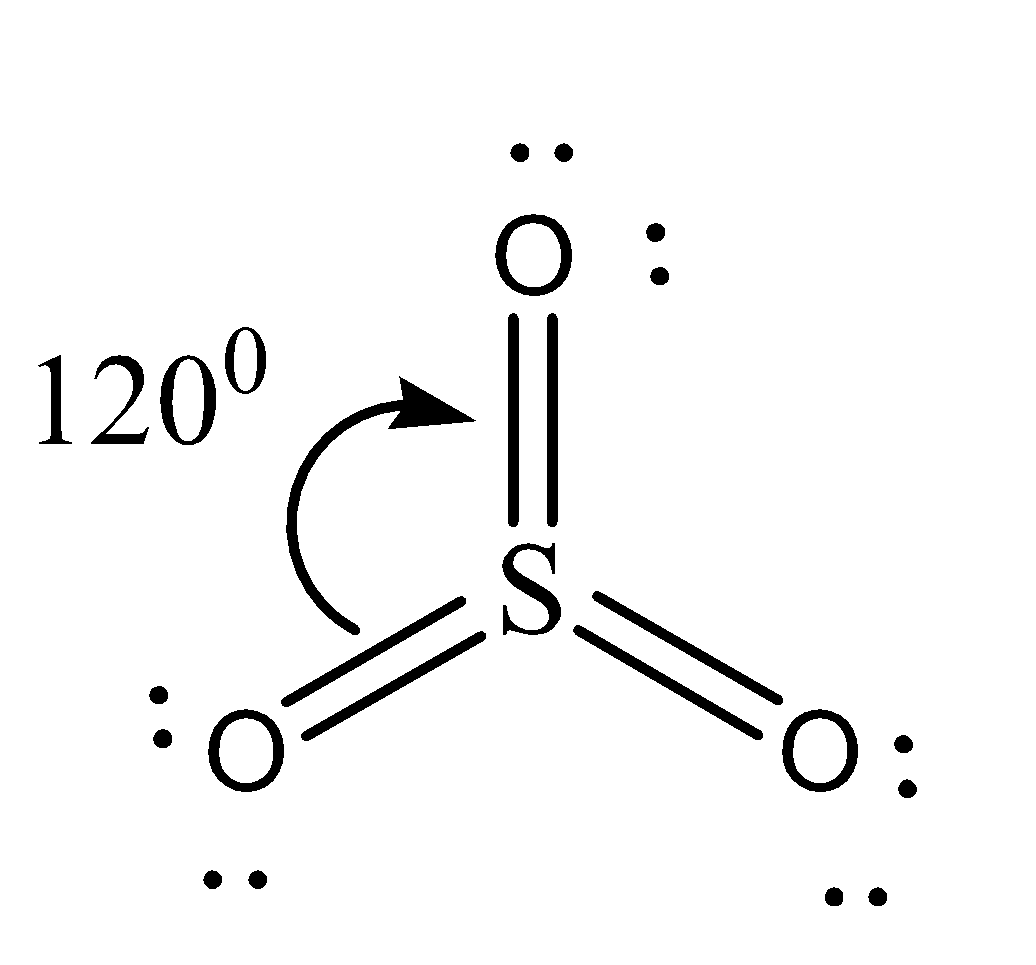
Option C is correct as the reason given is incorrect and the assertion is correct.
Note:
We must have to remember that the hybridization of sp2 is the combining of atomic orbitals of one s and two p, which includes the promotion of one electron to one of the atomic orbitals of 2p in the s orbital. Three new hybrid orbitals equal to the energy level are formed by the combination of these atomic orbitals.
Pi bonds are covalent chemical bonds where two orbital lobes on one atom overlap with two orbital lobes on another atom, and this overlap takes place laterally. Each of these atomic orbitals, moving through the two bonded nuclei, has zero electron density in a shared nodal plane.
