Question
Question: Assertion: P- Hydroxybenzoic acid has a lower boiling point than o- hydroxybenzoic acid. Reason: ...
Assertion: P- Hydroxybenzoic acid has a lower boiling point than o- hydroxybenzoic acid.
Reason: O- Hydroxybenzoic acid has intramolecular hydrogen bonding.
A. Both assertion and reason are true and reason is the correct explanation of assertion.
B. Both assertion and reason are true and reason is not the correct explanation of assertion.
C. Assertion is true and reason is false.
D. Assertion is false and reason is true.
Solution
To solve assertion reason type of questions, we will firstly look into the assertion and read it without looking at the reason. Once we read the question, then we will try to analyze its reason by yourself. Then we will look at the reason. If the reason is similar to what we thought, we will mark option A, and if not then we need to look for options B, C and D.
Complete step-by-step answer: This question is based on the concept of boiling point of phenol. In phenol we know that O and H are present and both have high difference in electronegativity. Also, water is a polar solvent. Therefore, there are maximum chances of hydrogen bonding between both the atoms.
Now, the assertion says P- Hydroxybenzoic acid has a lower boiling point than o- hydroxybenzoic acid. Let us analyze this statement.
We have already discussed that hydrogen bonding is possible in such acids as O and H have high differences in electronegativity. If you are able to recall, then we know that Hydrogen bonding are of two types.
1. Intermolecular hydrogen bonding.
2. Intramolecular hydrogen bonding.
Now, we should remember the fact that in any organic compound, if there is ortho substituent and hydrogen bonding is possible in that compound then always the bonding will be intramolecular hydrogen bonding. If the substituent is at para position, then intermolecular hydrogen bonding exists.
Therefore, we can say that O- Hydroxy benzoic acid will show intramolecular benzoic acid and P- Hydroxy benzoic acid will show intermolecular hydrogen bonding.
The hydrogen bonded structures are shown below:
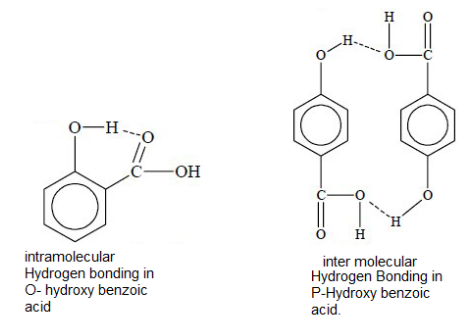
Now, we should remember that Intermolecular hydrogen bonding results in the association of molecules. Hence, it usually increases the melting point, boiling point, viscosity etc. Whereas intra molecular hydrogen bonding results in the cyclisation of molecules and thus prevents their association, as a result it decreases the boiling point, melting point.
Therefore, we can say that
P- Hydroxybenzoic acid because of inter molecular hydrogen bonding has a higher boiling point than o- hydroxybenzoic acid.
Hence, the assertion given in the question is wrong.
Now, if we look at the reason it states that O- Hydroxybenzoic acid has intramolecular hydrogen bonding. From the above explanation we can say that this statement is true.
So, we can analyze that assertion is false, but reason is true.
Hence, the correct option is option D.
Note: Hydrogen bonding has a marked influence on the properties of various substances. The compounds whose molecules are associated with hydrogen bonding have abnormally high melting and boiling points. It is because a large amount of energy is needed to overcome intermolecular hydrogen bonds and to separate the molecules.
