Question
Question: **Assertion:** \[{P_4}\] exists but \[{N_4}\] does not; \[{N_2}\] exists but \[{P_2}\] does not. *...
Assertion: P4 exists but N4 does not; N2 exists but P2 does not.
Reason:(White phosphorus: P4) Given : N≡N 946 kJmol−1, P≡P 481 kJmol−1, N−N 160 kJmol−1, P−P 215 kJmol−1 Greater the B.E., greater the stability [compare BE of N≡Nand N−N(in N4) and P−P(in P4)]
Read the above assertion and reason and choose the correct option regarding it.
A. Both Assertion and Reason are correct and Reason is the correct explanation for Assertion.
B. Both Assertion and Reason are correct and Reason is not the correct explanation for Assertion.
C. Assertion is correct but Reason is incorrect.
D. Both Assertion and Reason are incorrect.
Solution
White phosphorus is a colourless, white, or a yellow waxy solid possessing a garlic-like odour and has a tetrahedral structure. On the other hand, dinitrogen is a colourless, odourless and tasteless gas which makes up 78% (by volume) of the atmosphere.
Complete answer:
White phosphorus consists of P4molecules. Thus, white phosphorus exists as a P4 molecule in both solid as well as vapour states. Actually owing to larger atomic size P cannot form pi bonds (hence, possess less B.E.) and thus, it is a tetra-atomic molecule where each P atom is connected with 3 other P atoms via 3 sigma bonds. Actually, the outer shell electrons of P atoms are farther and separated due to its large size and hence, cannot form a triple bond. As a result, it forms bond with other three P atoms to complete its octet as shown below:
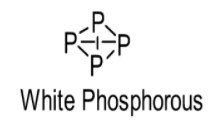
On the other hand, owing to the smaller atomic size N can form 1 sigma and 2 pi bonds which means a triple bond (hence, possess more B.E.) with another N atom and occurs as a diatomic molecule or dinitrogen (N2). In this case, the valence shell electrons are very close (owing to smaller size of atom) and have the tendency to form pi bond with another N atom as shown below:

**Hence, the correct answer is Option A i.e. Both Assertion and Reason are correct and Reason is the correct explanation for Assertion.
Note:**
Sigma bonds are much stronger than the pi bonds owing to the reason that atomic orbitals which form sigma bonds overlap to a greater extent compared to the orbitals which form pi bonds. Sigma bonds are mainly formed by head-on overlaps, whereas pi bonds are mainly formed by side-on overlaps.
