Question
Question: Assertion: Molecular formula of water is \({H_2}O\). Reason: One molecule of water contains two at...
Assertion: Molecular formula of water is H2O.
Reason: One molecule of water contains two atoms of hydrogen and one atom of oxygen.
(A) Both assertion and reason are correct and the reason is the correct explanation of assertion.
(B) Both assertion and reason are correct but the reason is not the correct explanation of assertion.
(C) Assertion is correct but the reason is incorrect.
(D) Both assertion and reason are incorrect.
Solution
We know that an atom is the smallest particle of an element and a molecule is a group of two or more atoms which are bonded together in an element or compound. If a molecule will contain atoms of different elements, then the substance will be known as compounds. So the molecular formula is the one which shows the number of each type of atom present in a molecule.
Complete answer: A water molecule is the one which consists of two atoms of hydrogen which are bonded to an oxygen atom through covalent bonds. We know that oxygen is more electronegative than hydrogen, so the oxygen atom acquires a partial negative charge and the two hydrogen atoms will acquire a partial positive charge. Therefore, water molecules are classified under the category of polar molecules.
Overall, the water molecule has a bent structure. This is because lone pairs are present on the oxygen atom so there will exist lone pair-bond pair repulsion between the oxygen and hydrogen atoms. Thus because of this repulsion force, the water molecule acquires a bent shape. The bond angle of water is observed to be 104.5∘
Structure of water molecule:
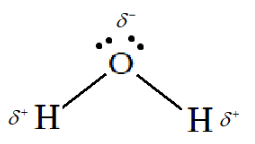
So, the correct answer is Option A. i.e. Both assertion and reason are correct and the reason is the correct explanation of assertion.
Note: We say that hydrogen and oxygen are bonded by a covalent bond as covalent bond is the bond formed by sharing of the valence electrons. So here we know that hydrogen needs only one electron and oxygen needs two electrons to complete their octet. Hence the two electrons of two hydrogen atoms bind with two electrons of one oxygen atom thereby forming a covalent bond.
