Question
Question: Assertion: Ionic bonds are always stronger than covalent bonds. Reason: They break only when bomba...
Assertion: Ionic bonds are always stronger than covalent bonds.
Reason: They break only when bombarded with electrons.
A.Both Assertion and Reason are correct and Reason is the correct explanation of Assertion.
B.Both Assertion and Reason are correct and Reason is not the correct explanation of Assertion.
C.Assertion is correct but Reason is not correct.
D.Assertion is not correct but Reason is correct.
E.Both the Assertion and reason are not correct.
Solution
Covalent bond is formed by sharing of electrons while ionic bond is formed by transfer of electrons. The presence of coulombic attraction between oppositely charged ions in ionic bonding leads to its stability.
Complete step by step answer:
Ionic bond involves complete transfer of electrons as there occurs formation of ions called cation and anion therefore, there exists a huge electrostatic force of attraction which makes it a strong bond. For example, sodium chloride is formed by an ionic bond between sodium and chloride ions.
Covalent bond involves sharing of two or more outer shell electrons that can hold all biomolecules together. Shared electrons are difficult to give away because two elements together share the electrons and make the bond stronger. For example, water molecules have covalent bonds in them.
We know that bond strength is inversely proportional to bond length. Bond length of ionic bonds is less as compared to that of covalent bonds. So we can say that ionic bonds are stronger than covalent bonds. Ionic bond is formed when there is small ionisation whereas covalent bonding occurs when polarisation degree is high.
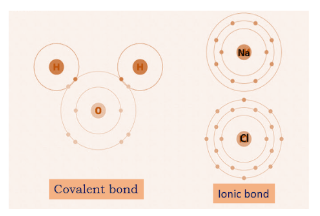
Since the presence of coulombic attraction between oppositely charged ions in ionic bonding leads to its higher stability, it is stronger than the covalent bonding.
Hence, the correct option is (C).
Note:
Polarisation power is the distortion in an ion due to other ion’s power is, is the phenomena responsible for covalent character in ionic bonding. This can be explained in detail by considering Fazan’s rules that even a 100% ionic bond has some characteristics of covalent bond.
