Question
Question: Assertion: Aniline can be prepared by the reaction of chlorobenzene with \(NaN{{H}_{2}}/Liq.N{{H}_{3...
Assertion: Aniline can be prepared by the reaction of chlorobenzene with NaNH2/Liq.NH3.
Reason: NH2− ion is a stronger nucleophile.
(A) Both Assertion and Reason are correct and Reason is the correct explanation for Assertion.
(B) Both Assertion and Reason are correct but Reason is not the correct explanation for Assertion.
(C) Assertion is correct but Reason is incorrect.
(D) Both Assertion and Reason are incorrect.
Solution
The formation of aniline takes place in presence of the strong base, that is, sodium amide which abstracts the proton, which is followed by the loss of the chlorine atom from the chlorobenzene compound. This makes the ring unstable and it gets attacked by the nucleophile.
Complete step by step solution:
In the mechanism for the reaction of chlorobenzene with sodium amide, NaNH2 in liquid ammonia. We see that the chlorine atom attached to the benzene ring makes the hydrogen on the adjacent carbon acidic.
The sodium amide which is a strong base abstracts this proton from the carbon adjacent to the chlorine, that is, at the ortho-position. The resulting anion formed, further loses the chlorine atom (the leaving group).
Due to the loss of the proton and the chloride ion, there are two sp2 orbitals present in the ring, which overlap and form a triple bond. An intermediate is formed called benzyne.
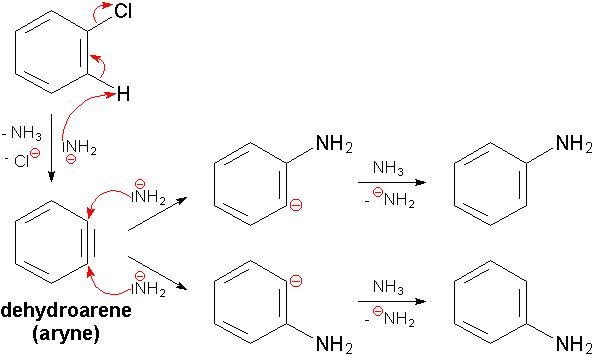
The benzyne is unstable and reactive. It acts as electrophilic in nature and gets attacked by the strong nucleophile (NH2−) present in the base (NaNH2/Liq.NH3).
Now this nucleophile (NH2−) can attack either side of the triple bond and the ammonia being a weaker acid donates its proton back to the benzene ring. So, we get a mixture of ortho- amino benzene and meta- amino benzene, that is, the aniline compound is formed.
Therefore, the formation of aniline by the reaction between chlorobenzene and sodium amide in liquid ammonia is satisfied by option- (B) Both Assertion and Reason are correct but Reason is not the correct explanation for Assertion.
Note: This is an elimination-addition reaction or a nucleophilic aromatic substitution reaction. As seen, the chloride ion (leaving group) is lost first and then the nucleophile (NH2−) gets attached. Also, the nucleophile is strong as it is a conjugate base of the ammonia. It should also be noted that the protons lose from the carbon adjacent to the leaving group on the benzene ring, so no reaction will occur if there is no hydrogen on the ortho-positions. Also, in this reaction, the benzyne is highly reactive because the triple bond formed is not from the overlap of the p-orbitals as in the alkynes but rather the sp2 hybrid orbitals that are perpendicular to the p-orbitals. So due to poor overlap, they are unstable.
