Question
Question: Assertion (A): The product of the reaction of organic compound I -CH₂- Br with NaCN is I -CH₂- CN....
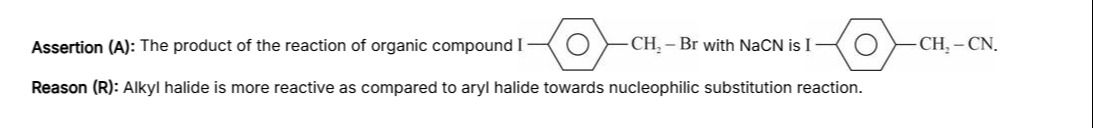
Assertion (A): The product of the reaction of organic compound I -CH₂- Br with NaCN is I -CH₂- CN.
Reason (R): Alkyl halide is more reactive as compared to aryl halide towards nucleophilic substitution reaction.
Both A and R are true and R is the correct explanation of A.
Both A and R are true but R is not the correct explanation of A.
A is true but R is false.
A is false but R is true.
Both A and R are true and R is the correct explanation of A.
Solution
Detailed analysis of the Assertion and Reason:
Assertion (A): The product of the reaction of organic compound Phenyl-CH₂- Br with NaCN is Phenyl-CH₂- CN.
- The reactant is benzyl bromide (Phenyl-CH₂-Br). In this compound, the bromine atom is attached to an
sp3hybridized carbon atom, which is further attached to a phenyl group. This is a benzylic halide, which behaves similarly to primary alkyl halides in nucleophilic substitution reactions, often with enhanced reactivity due to resonance stabilization. - The reagent is sodium cyanide (NaCN), which provides the cyanide ion (CN⁻) as a strong nucleophile.
- The reaction is a nucleophilic substitution reaction, specifically an SN2 reaction. The cyanide ion attacks the carbon bearing the bromine, displacing the bromide ion.
- The reaction proceeds as follows:
C₆H₅-CH₂-Br + NaCN → C₆H₅-CH₂-CN + NaBr - The product formed is benzyl cyanide (Phenyl-CH₂-CN).
- Therefore, Assertion (A) is true.
Reason (R): Alkyl halide is more reactive as compared to aryl halide towards nucleophilic substitution reaction.
-
Alkyl halides (R-X): In alkyl halides, the halogen atom is attached to an
sp3hybridized carbon atom. The C-X bond is a single bond and can be readily broken in nucleophilic substitution reactions (SN1 or SN2). -
Aryl halides (Ar-X): In aryl halides (e.g., bromobenzene, C₆H₅-Br), the halogen atom is directly attached to an
sp2hybridized carbon atom of the benzene ring. Their low reactivity towards nucleophilic substitution is due to several factors:- Resonance effect: The lone pair electrons on the halogen atom are delocalized into the benzene ring, giving the C-X bond partial double bond character. This makes the C-X bond stronger and shorter, and thus more difficult to break than a single C-X bond in alkyl halides.
- Hybridization state of carbon: The carbon atom to which the halogen is attached in aryl halides is
sp2hybridized. Ansp2hybridized carbon is more electronegative than ansp3hybridized carbon (as in alkyl halides), making the C-X bond less polar and holding onto the electrons more tightly, thus making it less susceptible to nucleophilic attack. - Instability of phenyl carbocation: If an SN1 mechanism were to occur, it would involve the formation of a phenyl carbocation (C₆H₅⁺), which is highly unstable because the positive charge is on an
sp2hybridized carbon and cannot be effectively stabilized by resonance.
-
Due to these reasons, aryl halides are significantly less reactive towards nucleophilic substitution reactions compared to alkyl halides. They require extreme conditions or different mechanisms (like nucleophilic aromatic substitution via addition-elimination or benzyne mechanism) to react.
-
Therefore, Reason (R) is true.
Relationship between Assertion (A) and Reason (R):
The compound in Assertion (A), benzyl bromide (Phenyl-CH₂-Br), is a benzylic halide. While not a simple alkyl halide, it behaves like an alkyl halide in terms of the C-Br bond being on an sp3 hybridized carbon, not directly on the aromatic ring. This allows it to undergo facile nucleophilic substitution. The Reason (R) correctly states that alkyl halides (and by extension, benzylic halides like benzyl bromide) are more reactive towards nucleophilic substitution than aryl halides. This difference in reactivity is precisely why the reaction in Assertion (A) proceeds readily under normal conditions, whereas an aryl halide (like bromobenzene) would not react similarly with NaCN. Hence, Reason (R) provides a correct and relevant explanation for the feasibility of the reaction described in Assertion (A).
The reaction in A is possible because the compound is a benzylic halide, which falls under the general category of halides that are reactive towards nucleophilic substitution, as opposed to aryl halides which are unreactive.
