Question
Question: Assertion (A): \({{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{CHO}}\) on heating with dilute...
Assertion (A): C6H5CHO on heating with dilute NaOH forms aldol condensation product.
Reason(R): C6H5CHO has no alpha-hydrogen.
A.Both A and R are true and R is the correct explanation of A
B.Both A and R are true and R is not the correct explanation of A.
C.A is true but R is false.
D.A is false but R is true.
Solution
Aldol condensation is an organic reaction in which enolate ion reacts with a carbonyl compound to form beta-hydroxy ketone or beta-hydroxy aldehyde. This is then followed by a dehydration process to give a conjugated enone. Aldol condensation is an important organic synthesis, to form a new carbon-carbon bond.
Complete step by step answer:
Mechanism for Aldol condensation reaction:
CH3CHONaOHH2O,ΔCH3CH = CHCHO
Step 1: Deprotonation of aldehyde by hydroxide ion
Step 2: The enolate ion formed in step 1 will add to the unreacted aldehyde.
Step 3: The alkoxide ion formed in step 2 will be protonated by water and will form Aldol.
Step 4: A small amount of Aldol is converted into enolate ion by the hydroxide ion.
Step 5: The enolate ion formed in the above step will lose a hydroxide ion and will form alpha-beta-unsaturated aldehyde.
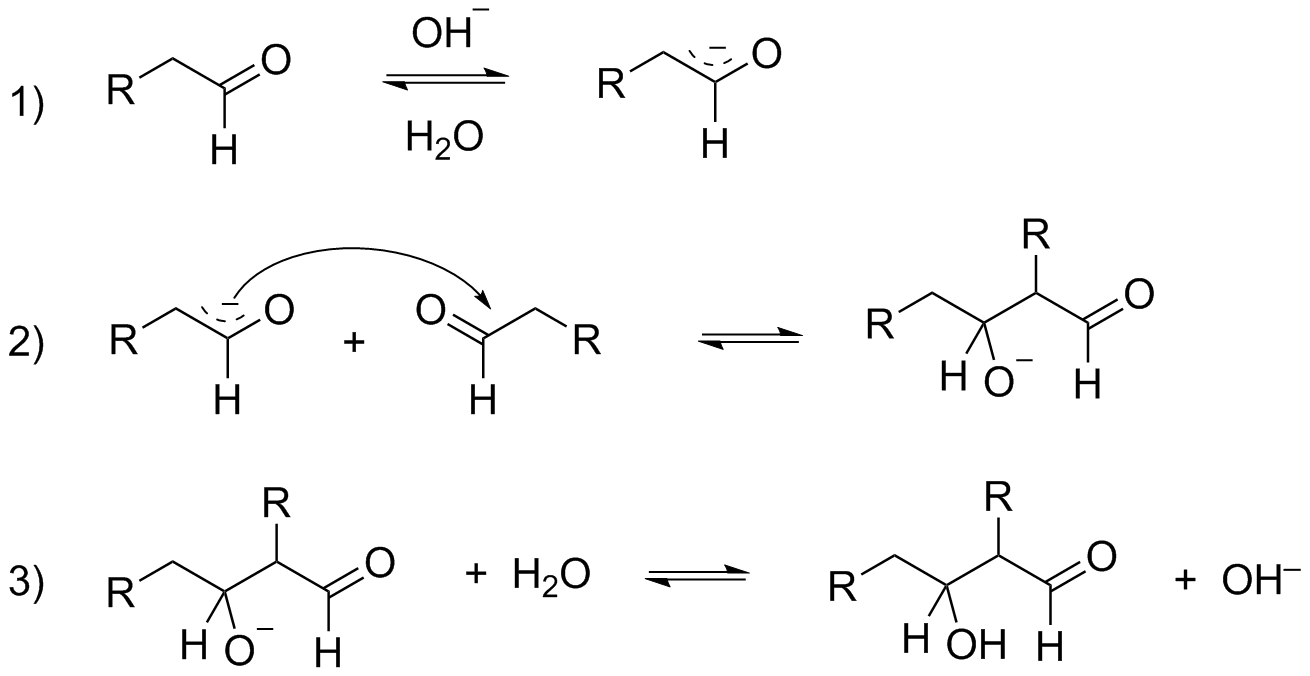
As you can notice in the mechanism of Aldol condensation presence of alpha-hydrogen is compulsory for the reaction to proceed. And when you look at C6H5CHO, there is no alpha-hydrogen and hence, the given compound will not undergo Aldol condensation.
Hence the assertion (A) is incorrect but the reason (R) is correct.
So, the correct answer is Option D .
Note:
Alpha-hydrogens are the hydrogens attached to the carbon atom next to the functional group. Now, as you have seen the presence of alpha-hydrogen is necessary for Aldol condensation. Compounds not having alpha-hydrogens undergo Clasien condensation reaction rather than Aldol condensation. Therefore, C6H5CHO will undergo a Claisen condensation reaction.
