Question
Question: Aspirin can be prepared by: A: Reimer-Tiemann reaction on phenol B: Friedel-Crafts acylation on ...
Aspirin can be prepared by:
A: Reimer-Tiemann reaction on phenol
B: Friedel-Crafts acylation on benzoic acid
C: Esterification of phenol using salicylic acid
D: Acetylation of salicylic acid using acetic anhydride in the presence of phosphoric acid
Solution
Hint : As we know that Aspirin, also known as acetylsalicylic acid (ASA), is a white, crystalline and a weakly acidic substance. It possesses a melting point of around 136∘C (or 277∘F ), and a boiling point of around 140∘C ( 284∘F ). It is generally prepared with the help of Salicylic acid and an acid anhydride.
Complete Step By Step Answer:
Let us talk about the structure of aspirin which is shown below:
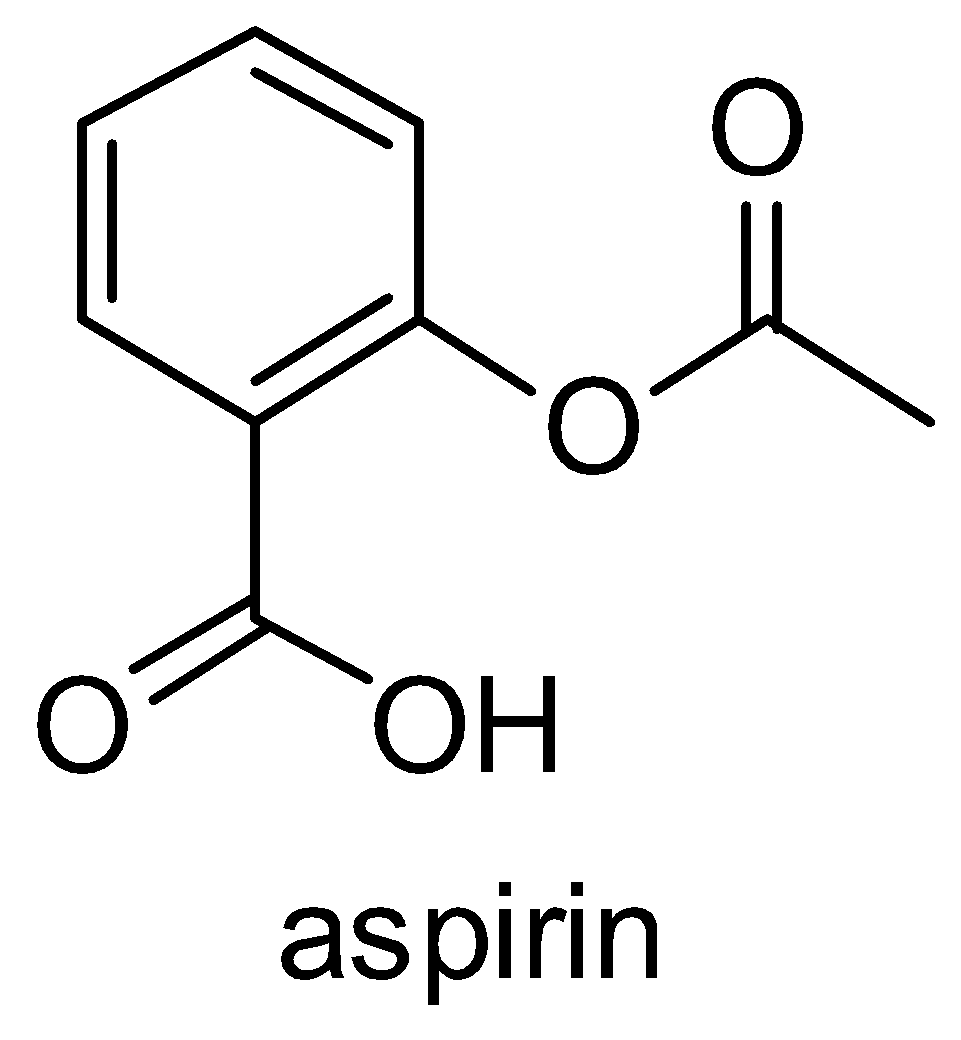
Now, we will discuss each reaction given in the options briefly:
Option A: we know that the Reimer–Tiemann reaction on phenol refers to a chemical reaction which is used for the ortho-formylation of phenols. Example: conversion of phenol into salicylaldehyde as depicted below:
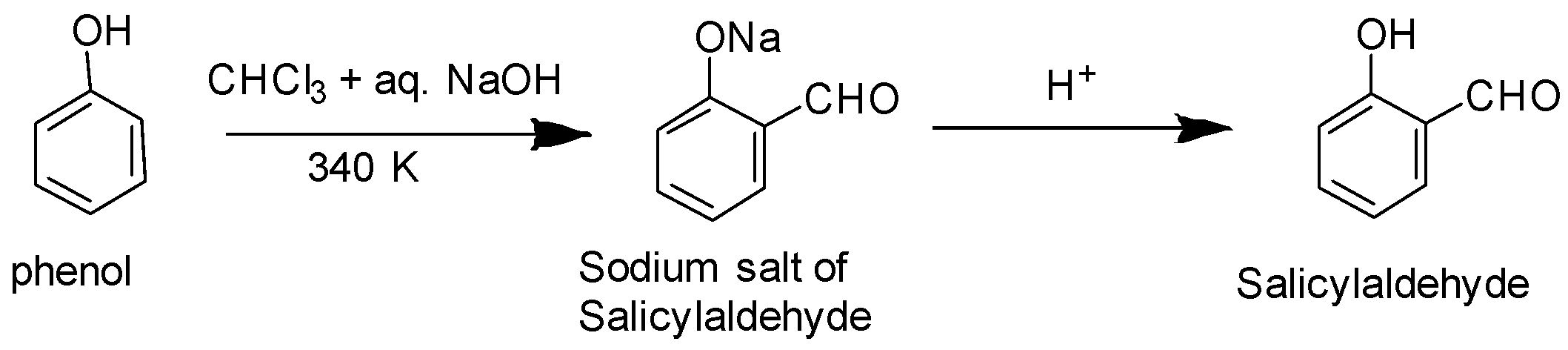
Option B: Benzoic acid cannot undertake Friedel Crafts reaction as the carboxyl group is strongly deactivating and the catalyst i.e. anhydrous aluminium chloride utilised in the reaction acts as a Lewis acid form bond with carboxyl group.
Option C: Esterification of phenol using salicylic acid leads to the formation of benzoate ester (i.e. phenyl salicylate or salol) as shown below:
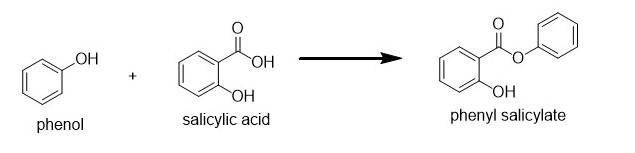
Option D: lastly we have Acetylation of salicylic acid using acetic anhydride in the presence of phosphoric acid leads to the production of acetylsalicylic acid (i.e. aspirin) and acetic acid as shown below:
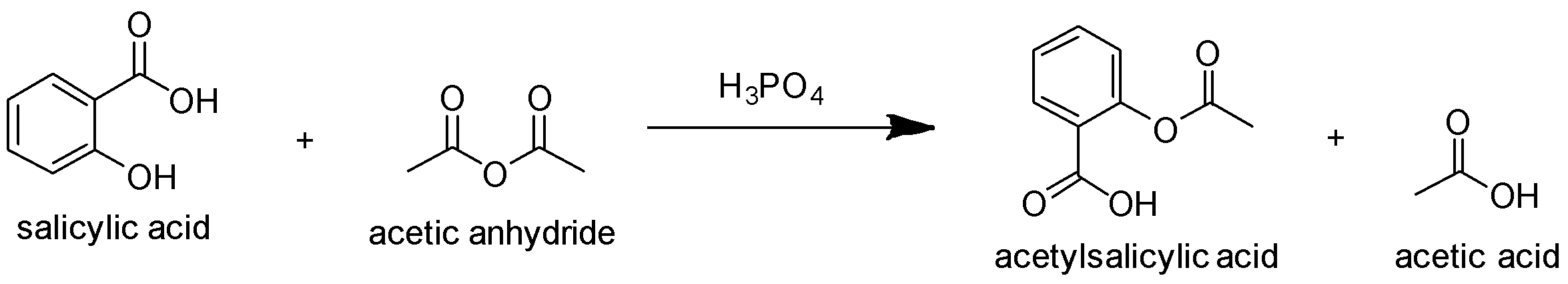
Hence, the correct answer is Option D.
Note :
Always remember that Aspirin is a NSAID i.e. non-steroidal anti-inflammatory drug. It is employed to reduce fever, pain or inflammation. Aspirin can even reduce the risk of death if given immediately after a heart attack. It may also be used to decrease the risk of cancer, specifically colorectal cancer.
