Question
Question: Aside from concentrated sulphuric acid, what other acid is employed in the dehydration of alcohols? ...
Aside from concentrated sulphuric acid, what other acid is employed in the dehydration of alcohols?
A)Concentrated hydrochloric acid
B)Conc. Acetic acid
C)Conc. Phosphoric acid
D)Conc. acetone
Solution
To answer this question, you should recall the concept of dehydration of alcohols. This dehydration of alcohols generates alkene and is performed by heating the alcohols in the presence of strong acids, such as sulfuric or phosphoric acid.
Complete Step by step solution:
We know that dehydration of alcohol is done using an acid catalyst. The acid catalysts which are generally used are either concentrated sulphuric acid or concentrated phosphoric (V) acid, H3PO4 . Phosphoric(V) acid is preferred over sulphuric acid because it is safer and produces a less messy reaction. Phosphoric(V) acid is a weaker oxidising agent compared to concentrated sulphuric acid.
Therefore, we can conclude that the correct answer to this question is option C. A reaction of alcohol is shown in the below figure using concentrated H3PO4
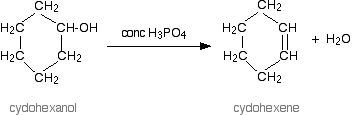
Note: You should know that alcohols are amphoteric. The lone pair of electrons on oxygen makes the -OHgroup a weak base. In this molecule, the oxygen can donate two electrons to an electron-deficient proton. This means that in the presence of a strong acid, R–OH acts as a base and protonates into the very acidic alkyloxonium ion. This weakly basic nature of alcohols is essential for its dehydration reaction with an acid to form alkenes. The temperature range for each substituted alcohol is different:
1∘: 170∘ − 180∘C
2∘:100∘− 140 ∘C
3∘: 25∘− 80∘C
