Question
Question: Arrange the following (w, x, y, z) in decreasing order of their boiling points:  in decreasing order of their boiling points:
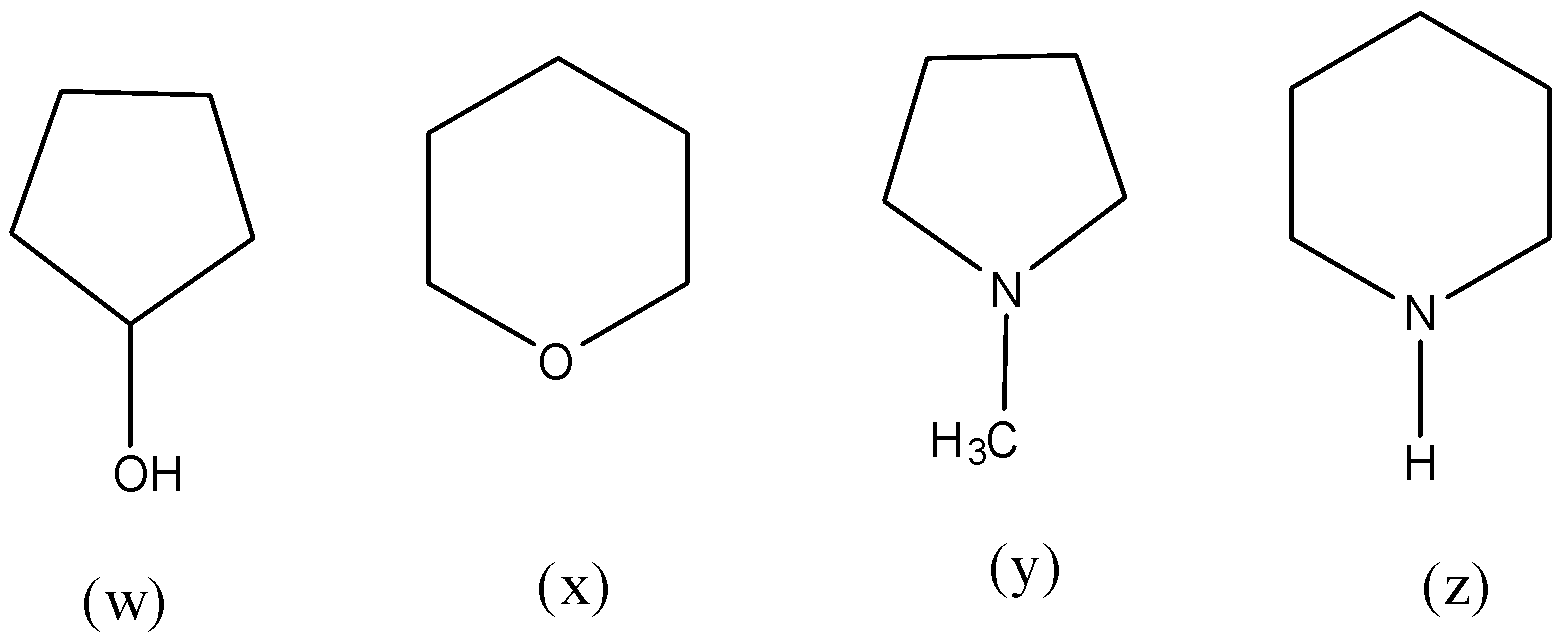
A. w > x > z > y
B. w > x > y > z
C. w > z > y > x
D. w > z > x > y
Solution
If the molecules are having hydrogen bonding (inter molecular hydrogen) in between them then the boiling point increases. The boiling point also depends on the electronegativity of the atoms present in the given molecules.
Complete Solution :
- In the question it is given that to write the order of boiling points of the given molecules.
- We know that boiling point is directly proportional to the presence of the hydrogen bonding.
- The structures ‘w’ and ‘z’ contain alcohol and amine functional groups in their structures.
- Means structure ‘w’ and ‘z’ has more boiling points due to the presence of the hydrogen bonding.
- The electronegativity of the oxygen atom is more when compared to electronegativity of the nitrogen atom.
- So, the boiling point of ‘w’ is greater than the boiling point of the ‘z’ ( w > z).
- Now coming to structures ‘x’ and ‘y’.
- The structure ‘x’ has more boiling points than the structure ‘y’ due to the presence of more electronegative oxygen atoms in the structure of ‘x’ ( x > y).
- Therefore the decreasing order of boiling points of the given compounds is as follows.
w > z > x > y
So, the correct answer is “Option D”.
Note: The boiling point of the organic compounds is going to depend on the inter molecular hydrogen bonding of the compounds and the electronegativity value of the atoms which are present in the organic compounds.
