Question
Question: Arrange the following (w, x, y, z) in decreasing order of their boiling points:  in decreasing order of their boiling points:
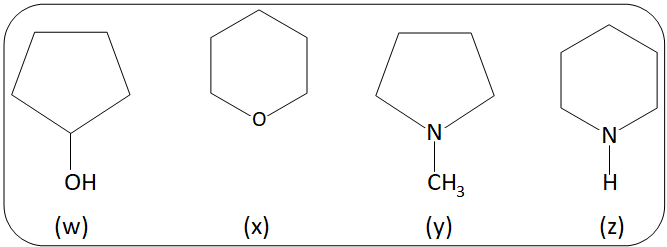
Solution
Hint- In order to solve this question, we will first see the properties on which the boiling point of a compound depends upon. On the basis of that property we will compare the boiling point of the given compounds. In this case we will check for the surface area and molecular mass, as the boiling point depends upon them.
Complete answer:
As we know that boiling point is proportional to the extent of H bonding and the surface area of the molecule.
As the H bonding is much higher in oxygen atom in comparison with nitrogen atom and if the surface area or molecular mass of compound is higher than the boiling point of that compound will also be greater.
In compound w H- bonding of oxygen atom is much higher in comparison with compound z so the boiling point w of is much greater than to z .
⇒w>z
And the surface area or molecular mass of compound x is higher than compound y so the boiling point of x is higher in comparison with y .
⇒x>y
As there are no hydrogen bonds in compounds x&y so, the boiling points of x&y will be lower than that of w&z
Hence, the decreasing order of their boiling points are w>z>x>y
So, the correct answer is option D.
Note- A liquid at higher pressure has a higher boiling point than when the atmospheric pressure of that liquid is lower. The regular boiling point of a compound is an indicator of the compound's volatility. The higher the boil, the less volatile the compound is. There are various factors affecting boiling point of organic compounds, some of them are: strength of intermolecular forces, Length of carbon-carbon chain, branching and polarity.
